Figures & data
Figure 1. Schematic representation of thyroid hormone disruption by endocrine disruptors. Thyrotropin-releasing hormone (TRH) is produced by the hypothalamus and sensed by the anterior pituitary gland where the thyroid-stimulating hormone (TSH) is produced and released into the general circulation. TSH promotes the secretion of thyroxine (T4) and triiodothyronine (T3) from the thyroid gland. Thyroid follicular cells uptake iodine (I-) from the circulation by sodium/iodide symporter (NIS), iodine will be later used for the production of thyroid hormones. NIS is blocked by CIO4-. A negative feedback loop for T4 and T3 is mediated by the sensing of circulation T4 and T3 levels by the anterior pituitary and the hypothalamus. Pesticides, Polychlorinated biphenyls (PCBs), phthalates and bisphenol A (BPA) can interfere with thyroid hormone signaling and affect circulating levels of T4 and T3. In the central nervous system, T4 crosses the brain-blood barrier (BBB) and is taken up by astrocytes via OATP1C1. Once inside the astrocytes, T4 is converted to T3 by the action of D2. T3 exits the astrocytes via MCT8 and enters oligodendrocytes and neurons via the same MCT8 transporter.
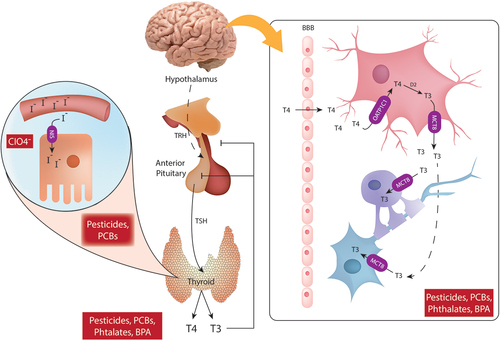
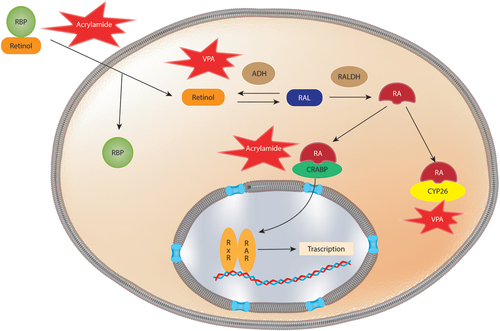
Figure 2. Schematic representation of Retinoic acid (RA) signaling disruption mediated by xenobiotics. Retinoid binding protein (RBP) facilitate the cellular uptake of retinoids. Once inside the cell, the conversion of retinol to RA is mediated by alcohol dehydrogenases (ADH) and retinaldehyde dehydrogenases (RALDH). Cyp26 metabolized RA into the less bioactive 4-oxo-retinoic acid. Retinoic acid binding proteins (CRABPs) transport RA into the nucleus where RA binds to corresponding response elements. Acrylamide has been shown to downregulate the expression of Crabp2 and Rpb7, likewise VPA downregulates Cyp26a1 and affects Aldh1a2 altering RA signaling.