Figures & data
Figure 1. Tle1Vc is a lipase effector with antibacterial activity.
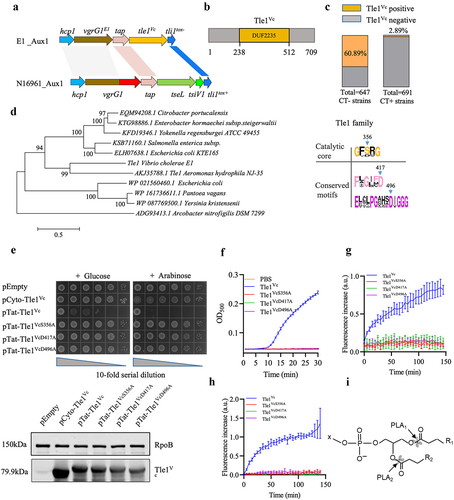
Figure 2. Tle1Vc effector impairs the integrity of bacterial membrane.
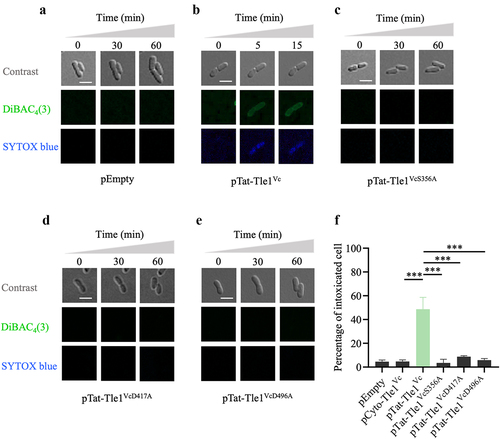
Figure 3. Tli1tox- specifically neutralizes the toxicity of Tle1Vc.
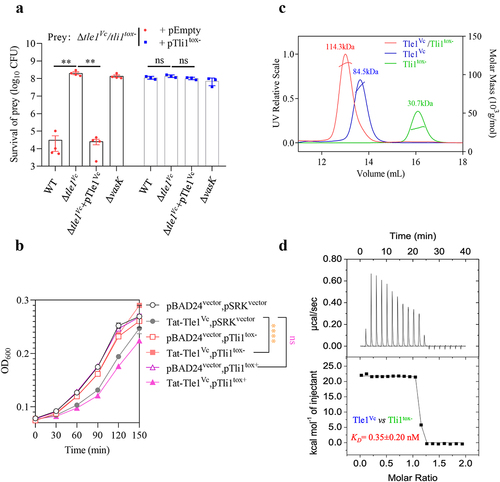
Figure 4. Tle1Vc delivery requires functional T6SS and VgrG1E1.
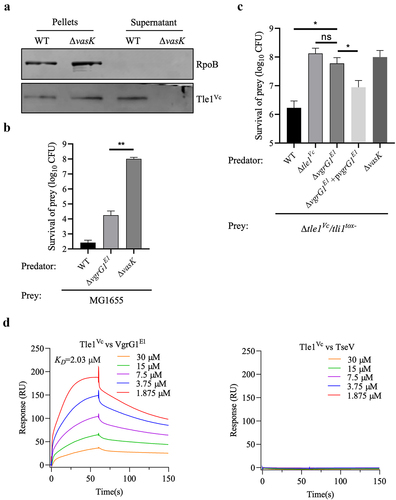
Figure 5. Tle1Vc contributes to Vibrio cholerae intra- and interspecies antagonism.
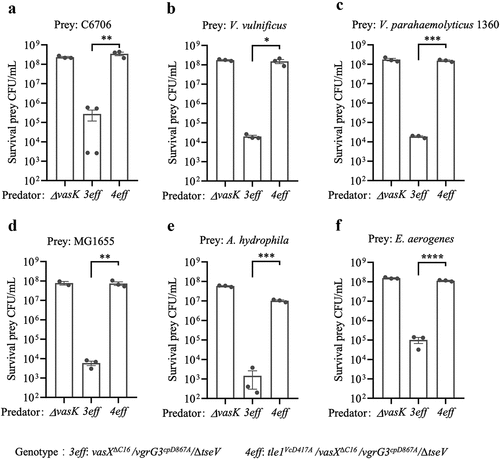
Figure 6. Tle1vc-induced strong tit-for-tat response requires the T6SS-H1 of PAO1, catalytic activity of effector and the physical puncture of T6SS.
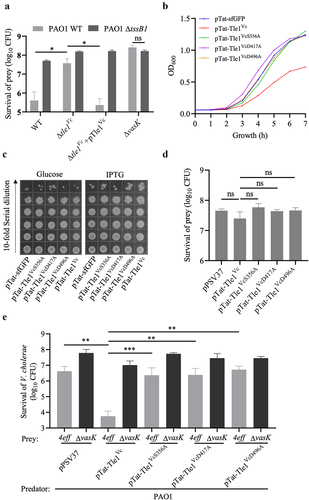
Figure 7. Phospholipase effector Tle1Vc mediates bacterial motility through modulating flagellar gene expression.
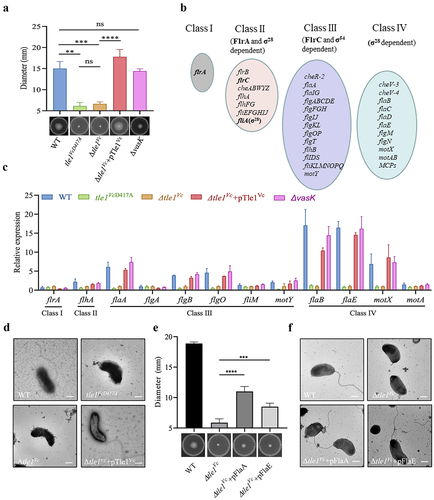
Figure 8. The effect of Tle1Vc on E1’s in vivo fitness and survival of immunity mutant.
Supplemental Material
Download Zip (3.8 MB)Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
