Figures & data
Table 1. Electronic database search algorithm.
Figure 1. Virion structure and genome organization of Marburg virus. Top, the Marburg virus structure along with depicting the structural proteins. Bottom, an illustration of the genome organization of the Marburg virus. This seven-gene strain of Marburg virus has been drawn roughly to scale. The light blue boxes indicate noncoding areas, as well as the colored box code regions for genes. The red arrows demonstrate the position of the transcriptional start signals, whilst the pale brown bars highlight conserved transcriptional stop signals. The genes are segregated by intergenic regions, indicated using black arrows, with the exception of the overlapping sequence (black triangle) between VP24 and VP30. At the extreme ends, the 3' and 5' trailer sequence is shown.
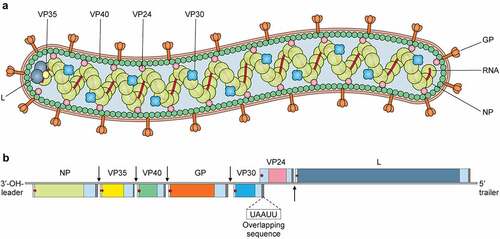
Table 2. Marburg virus genes, proteins and their characteristics and functions.
Table 3. Marburg virus outbreaks epidemiology and case fatality rate (CFR) in accordance with the outbreak strain.
Figure 2. Outbreak history of Marburg virus. The red color on the map demonstrates the occurrences of outbreaks associated with new strains of the Marburg virus. Primarily, MARV outbreaks have been identified in four countries in Africa: The Democratic Republic of Congo, Angola, Kenya, and Uganda. However, an outbreak in Zimbabwe was also associated with a novel strain of the Marburg virus, which caused an outbreak in South Africa. The yellow color on the map shows infections in which the source is known to have originated from another country. This type of outbreak occurred in Germany, the Netherlands, the USA, and South Africa. The green color shows the outbreaks associated with unintentional laboratory exposures. This sort of epidemic took place only in Russia.
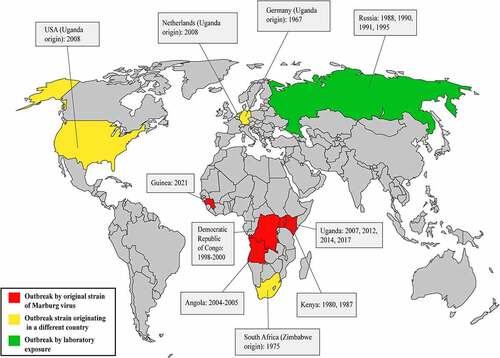
Figure 3. Number of MARV strains identified from reservoir bat species, which were responsible for the disease spread in different years and countries.
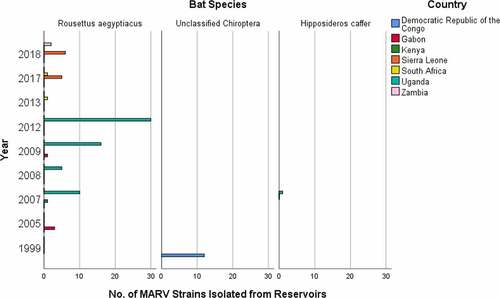
Figure 4. Transmission and spread of Marburg virus. Reservoirs of the Marburg virus, such as African fruit bats, can spread the virus among themselves by direct contamination, through sexual transmission, or due to biting. Direct contact with reservoir hosts or viral-contaminated fruit consumption may spread the virus to humans and non-human primates (NHPs). Transmission between humans and NHPs may occur through direct contact, and NHP-to-human transmission occurs due to bushmeat consumption and through direct contact. Direct contact and aerosols can facilitate both human-to-human and NHP-to-NHP transmission.
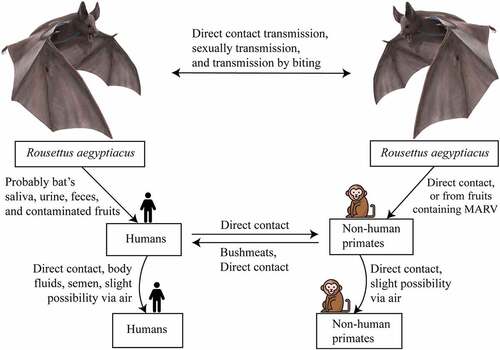
Table 4. Sources and infection types of Marburg virus.
Table 5. MVD symptoms, according to the phase of infection.
Figure 5. MARV entry, viral dissemination, and cellular tropism. The yellow color in the figure shows viral entry mechanisms, whereas the red color shows viral dissemination pathways. MARV enters the host and spreads throughout the lymphatic and vascular systems. The light brown color indicates the damaged cellular organelles. MARV causes necrosis in many organs, including the liver, spleen, kidneys, gastrointestinal tract, and endocardium.
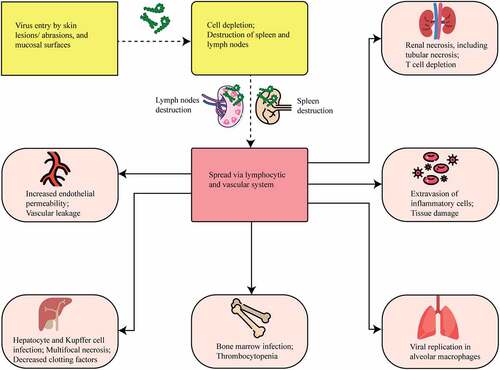
Figure 6. MARV hemorrhagic fever pathophysiology model in humans. Marburg virus primarily targets dendritic cells, monocytes, parenchyma cells at a liver, adrenocortical cells, and several lymphoid tissues. Infection of dendritic cells leads to poor stimulating condition of T lymphocyte that causes lymphocyte apoptotic condition. Due of this, body’s immunity is suppressed and cytokines/chemokines number is increased, which leads to shock as well as multiorgan damaging occurrence. Macrophage or monocyte infection leads to uncontrolled cytokines/chemokines activation, and they continue the damaging of T lymphocyte and endothelial cell. Endothelial cell infection causes increase of blood vessels permeability and DIC (disseminated intra-vascular coagulopathy), while both occurrences lead to hemorrhages. Systemic replication can also occur because of this infection in endothelial cells. Parenchymal cell infection occurrences in liver can decrease coagulation factors, and these occurrences can cause hemorrhages later on. Adrenocortical cells of adrenal gland infecting occurrence by MARV can lead to disorders in the metabolism and dysregulated blood pressure; and hemorrhage occurs at a later stage due to these infections. MARV infection the on lymphoid tissues of lymphatic system, especially lymph nodes and spleen infections lead to tissue necrosis and malfunctioning adaptive immunity. Shock and lymphoid organ damage can occur in the later stage.
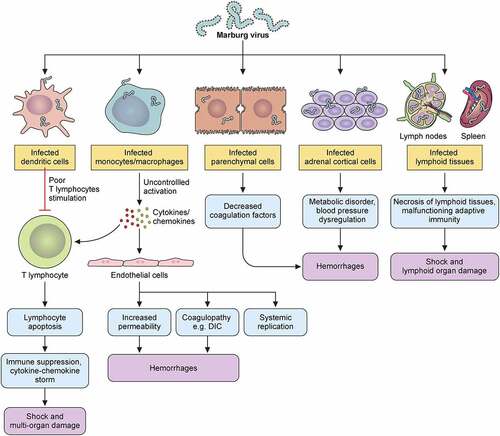
Table 6. Evaluation of Marburg virus treatment in NHP models.
Supplemental Material
Download MS Excel (77 KB)Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article [and/or] presented in the table and figures.
