Figures & data
Figure 1. Mutation of sarA in LAC attenuates virulence in a murine osteomyelitis model to a greater extent than mutation of any other regulatory locus. a) Femurs were harvested from mice 14 days post-infection and imaged by µct. Top and bottom panels are randomly chosen images from each experimental group obtained in two independent experiments. Numbers below each panel denote the percentage of broken bones observed in each experimental group. B and C) Quantitative analysis of cortical bone destruction (b) and new bone formation (c) of intact bones. d) Bacterial burdens were determined by homogenization of fractured and unfractured bones and serial dilution of the homogenates prior to plating on TSA. Results are reported as the average ± the standard error of the mean (SEM). e) Overall osteomyelitis (OM) scores were calculated to allow consideration of intact and broken bones. In all cases, statistical significance was assessed by one-way ANOVA. Numbers indicate p-values by comparison to the results observed with mice infected with the LAC parent strain.
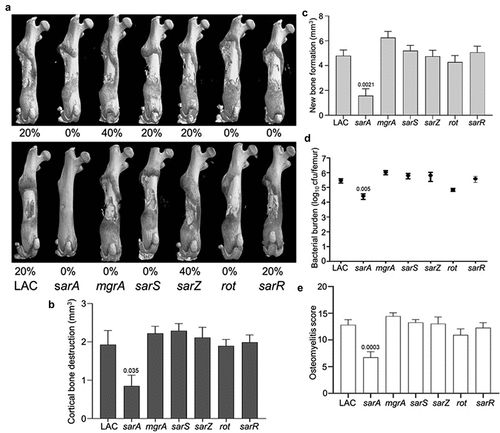
Figure 2. Mutation of sarA in LAC has the greatest effect on osteomyelitis related phenotypes owing primarily to its impact on protease production. a and b) Cytotoxicity of conditioned medium (CM) from overnight cultures of LAC and isogenic mutants was assessed using MC3T3-E1 (a) and RAW 264.7 cells (b) as surrogates for osteoblasts and osteoclasts, respectively. CM, sterile bacterial culture media (negative control), or ethanol (positive control) was mixed in a 1:1 ratio with the appropriate cell culture medium. Wells were stained with calcein-AM LIVE/DEAD Viability/Cytotoxicity Kit (Thermo Fisher Scientific). Viability is reported as fluorescence intensity/100,000. c) Biofilm formation was assessed using a microtiter plate assay and is reported as the absorbance of crystal violet staining at 595 nm (OD595). The impact of mutating rot on biofilm formation was statistically different from that of mutating sarA (p = 0.0214). d) for each strain, 1 x 106 colony forming units (cfu) was inoculated into TSB with or without 10 μg/mL indolicidin and incubated overnight with shaking. Growth was measured by the optical density at 600 nm (OD600) relative to a DMSO control for each mutant. e) 1 x 105 cfu of bacterial cells from exponential phase cultures were mixed with 1.0 ml of whole human blood. A sample was taken immediately after mixing and after a 3 hr incubation. Percent survival of each mutant was calculated and standardized relative to the results observed with LAC. The difference between the results observed with the sarA mutant and its protease-deficient derivative were not statistically significant. In all cases, statistical significance was assessed by one-way ANOVA. Numbers indicate p-values by comparison to the results observed with the LAC parent strain.
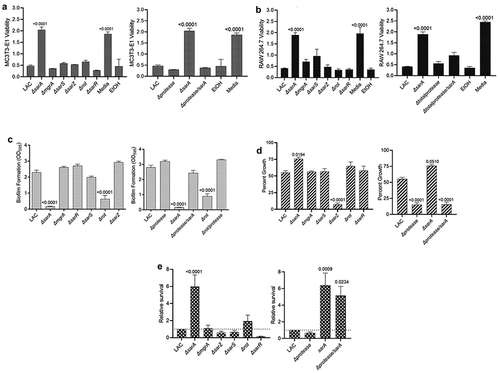
Figure 3. Mutation of sarA, mgrA, or rot in UAMS-1 results in attenuation in a murine osteomyelitis model. a) Femurs were harvested from mice 14 days post-infection and imaged by µct. Top and bottom panels are randomly chosen images mice from each experimental group obtained from two independent experiments. Numbers below each panel denote the percentage of broken bones observed in each experimental group. b and c) Quantitative analysis of cortical bone destruction (b) and new bone formation (c) of intact bones. d) Bacterial burdens were determined by homogenization and plating after µct and reported as the average ± the standard error of the mean (SEM). No statistically significant difference was observed in bacterial burdens with the sarA mutant, but a downward trend was evident (p = 0.0526). e) Overall osteomyelitis (OM) scores were calculated to allow inclusion of both intact and broken bones. In all cases, statistical significance was assessed by one-way ANOVA. Numbers indicate p-values by comparison to the results observed with mice infected with the UAMS-1 parent strain.
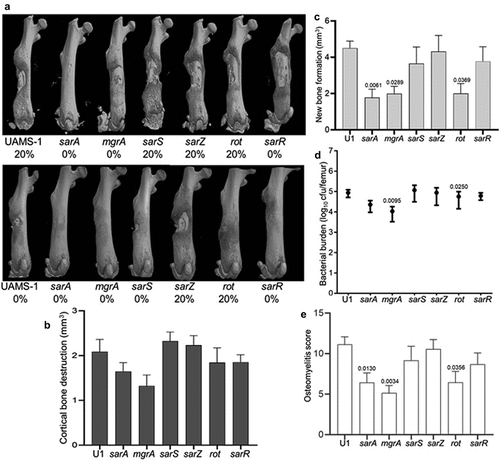
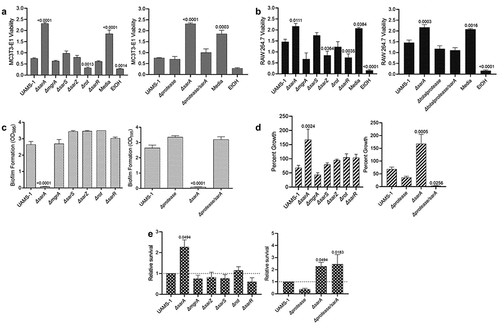
Figure 4. Mutation of sarA in UAMS-1 has the greatest effect on osteomyelitis-related phenotypes owing primarily to its impact on protease production. a and b) Cytotoxicity of CM from overnight cultures of UAMS-1 and isogenic mutants was assessed using MC3T3-E1 (a) and RAW 264.7 cells (b) as surrogates for osteoblasts and osteoclasts, respectively. CM, sterile bacterial culture media (negative control), or ethanol (positive control) was mixed in a 1:1 ratio with the appropriate cell culture medium for cytotoxicity assays. Wells were stained with calcein-AM LIVE/DEAD Viability/Cytotoxicity Kit (Thermo Fisher Scientific). Viability is reported as fluorescence intensity/100,000. c) Biofilm formation was assessed using a microtiter plate assay and is reported as the absorbance of crystal violet staining at 595nm (OD595). d) for each strain, 1 x 106 cfu was inoculated into TSB with or without 10 μg/mL indolicidin and incubated overnight with shaking. Growth was measured by OD600 relative to a DMSO control for each mutant. e) 1 x 105 cfu of bacterial cells from exponential phase cultures were mixed with 1 ml of whole human blood. A sample was taken immediately after mixing and after a 3 hr incubation. Percent survival of each mutant was calculated and standardized relative to the results observed with UAMS-1. The difference between the results observed with the sarA mutant and its protease-deficient derivative were not statistically significant. In all cases, statistical significance was assessed by one-way ANOVA. Numbers indicate p-values by comparison to the results observed with the UAMS-1 parent strain.
Supplemental Material
Download MS Excel (27.8 KB)Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.

