Figures & data
Figure 1. Triggers of myocarditis. Myocarditis can be induced by both infectious and non-infectious pathogens, with viral infection being the most common cause (red background).
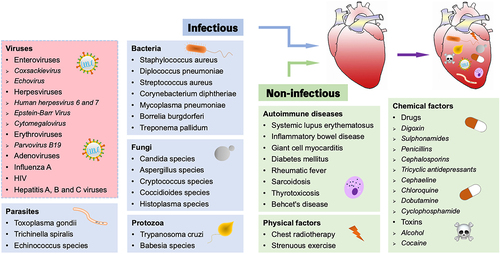
Figure 2. Temporal stages of CVB3-induced myocarditis. The first stage occurs when the virus enters into the host and translocates to the myocardium, causing various cellular responses and activating the host’s innate immunity for 1–7 d (green solid curve). The cellular and humoral responses lead to autoimmune-mediated damage in the second stage, with T lymphocyte infiltration cresting at 7–14 d, which typically lasts 7–28 d (blue solid curve). In certain patients, the inflammation fades as myocardial damage diminishes; nevertheless, in others, the virus stays in the body for months or years, giving rise to chronic inflammation and DCM (the third stage, red dotted curve).
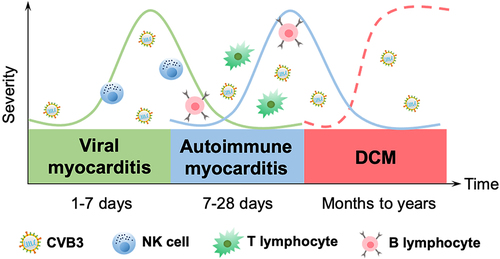
Figure 3. The immune mechanisms in the development of CVB3-induced myocarditis. Viral myocarditis is attributed to a combination of viral and host factors in vulnerable people. As viruses replicate, they trigger a cascade of PRRs (TLRs, NLRs, RAGEs) that are essential for activating immune cells and mediating the production of cytokines; simultaneously, NK cells directly destroy virally infected cells. In some cases, the virus will be eradicated during this process; otherwise, it can progress to chronic myocarditis or DCM with adaptive immunity engaged. The release of viral antigens is then followed by delivery through APCs (e.g. dendritic cells) that activate CD4+ T cells to develop into distinct subsets and secrete cytokines, whereas CD8+ T cells differentiate into CTLs that lyse virally infected cells. B cells secrete antibodies, and with the induction of anti-viral T cells and antibody responses, the infectious virus may be eradicated. After viral lysis of the infected cells, intracellular proteins (i.e. cardiac myosin) or cryptic epitopes (i.e. host antigens), are released and presented by APCs to CD4+ T cells, CD8+ T cells and B cells, which mediate inflammation through the secretion of cytokines, cytolysis, and production of autoantibodies, respectively, triggering an autoimmune response that in turn infiltrates the heart and exacerbates inflammation. Eventually, if the virus clears, the myocardium can revert to normal, but delayed or ineffective viral clearance generates myocyte degeneration, interstitial fibrosis, hypertrophy, and DCM.