Figures & data
Figure 1. F. nucleatum increased along with CRC progression and AI-2 accumulation (a) Representative images of FISH detecting F. nucleatum in CRC and NC tissues. (b) qRT-PCR data showed the significantly increased abundance of F. nucleatum in CRC tissues (n = 40) compared with that in NC tissues (n = 40). The relative abundance of F. nucleatum in tissues was estimated by qRT-PCR using the specific primers targeting F. nucleatum, and primers targeting gapdh were used to confirm the equal quantity of tissues. (c) qRT-PCR data showed the increased abundance of F. nucleatum along with the cancer progression. (d) the concentration of AI-2 is significantly higher in CRC tissues compared with NC tissues, and (e) increased along with the cancer progression. (f) Pearson’s correlation analysis showed a positive correlation between F. nucleatum abundance and AI-2 concentration in tissues of CRC patients.
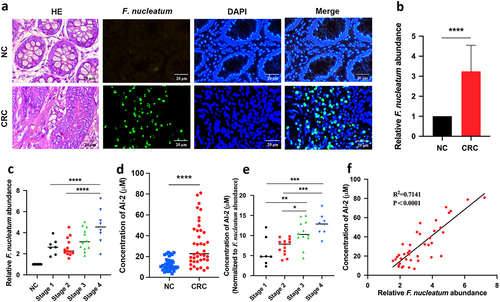
Figure 2. NE increased with the progression of F. nucleatum related CRC (a) the concentration of NE was significantly higher in CRC tissues (n = 40) compared with NC tissues (n = 40). (b) the concentration of NE in tumours increased along with the progression of CRC. (c, d) Pearson’s correlation analysis showed positive correlation between NE concentration and F. nucleatum abundance (c) or AI-2 concentration (d) in CRC tissues.
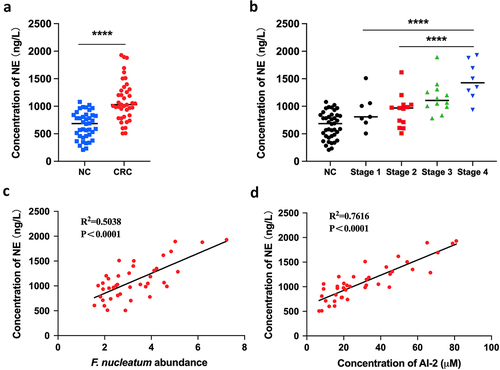
Figure 3. NE promoted the virulence and proliferation of F. nucleatum with upregulation of QS system (a) F. nucleatum was treated with NE for 24 hours followed by subjection to RNA-Seq. KEGG analysis of pathway enrichment showed the induction of QS after NE treatment. (b) volcano plot of the transcriptome in NE group versus NC group. QS associated genes (ccfA, ABC.PE.S, oppA, crp, blcC) and DNA replication associated genes (holB, dnaG, dnaN) were labelled. (c) Heatmap of differentially regulated genes associated with QS and DNA replication. (d) Representative images of the colony formation of F. nucleatum subjected to NE and propranolol treatments. (e) statistics of F. nucleatum density based on the colonies in (d). (f) qRT-PCR data showed that fadA expression was increased significantly with treatment of NE, and decreased by propranolol. All experiments were repeated three times, and one-way analysis of variance was used.
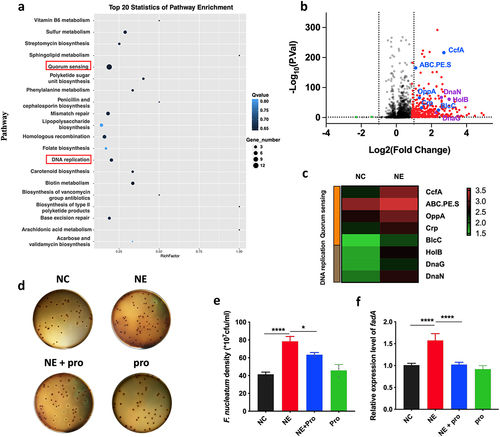
Figure 4. AI-2 is essential for NE enhanced virulence and proliferation of F. nucleatum (a) statistics of AI-2 concentration in the supernatant of F. nucleatum culture induced by NE. (b) Representative images illustrating the colony formation of F. nucleatum subjected to NE treatments with and without AI-2 inhibitor. (c) statistics of F. nucleatum density based on the colonies in (b). (d) qRT-PCR data showed that D-Rib significantly inhibited the FadA expression of F. nucleatum induced by NE. All experiments were repeated three times, and paired t test was used.
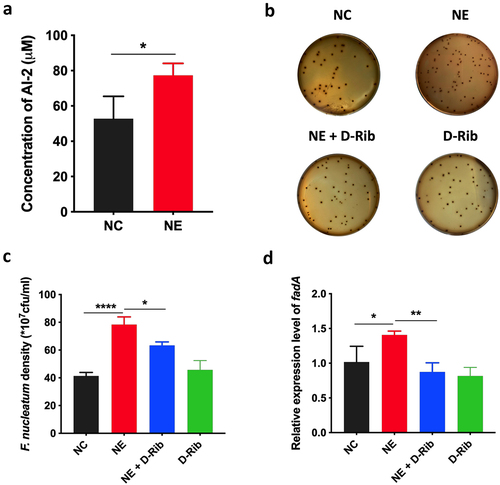
Figure 5. Stress promoted the progression of colorectal tumour in ApcMin/+ mice fed with F. nucleatum (a) Representative images showing the tumor formation and F. nucleatum invasion in tumours. (b, c) quantification of tumour number and size in each group. D-Rib significantly decreased the tumour number and size in mice fed with F. nucleatum. the tumour size in F. nucleatum + stress group was significantly higher than in F. nucleatum group. Both the tumour number and size were decreased significantly by catecholamine inhibitor AMPT. (d) qRT-PCR analysis highlighting F. nucleatum burden in tumours. AMPT significantly decreased the abundance of F. nucleatum induced by host stress. (e) F. nucleatum administration significantly increased AI-2 level in tumours, which was inhibited by D-Rib or AMPT.
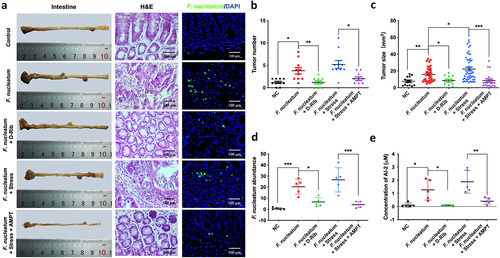
Fig S4.tiff
Download TIFF Image (606.5 KB)Fig S1.tif
Download TIFF Image (14.1 MB)Fig S2.tiff
Download TIFF Image (84.6 KB)Supplementary table S1.docx
Download MS Word (19 KB)Fig S3.tif
Download TIFF Image (3.6 MB)Data Availability statement
The authors confirm that the data supporting the findings of this study are available in the article and its supplementary materials.
