Figures & data
Figure 1. Overexpression of plastin 3, which is known to confer organization of actin microfilaments into bundles, perturbs the Sertoli cell TJ-permeability barrier function. (A) Treatment regimen used to transfect Sertoli cells with a full-length plastin 3 cDNA using the mammalian expression vector pCI-neo (pCI-neo/plastin 3) vs. pCI-neo (i.e., empty vector, control) on day 2 for 6 hr, cells were then rinsed twice to remove the transfection reagent. Thereafter, cells were cultured in fresh F12/DMEM until day 5 (i.e., 3 days after transfection for plastin 3 overexpression) before terminated for immunoblotting, or cell staining by fluorescence microscopy using different marker proteins. TER measurement to assess the TJ-barrier integrity was performed daily until day 8. (B) Immunoblotting that confirmed overexpression of plastin 3 in cultured Sertoli cell in vitro had no effects on the expression of selected BTB-associated proteins. (C) Overexpression of plastin 3 (plasmid DNA, both pCI-neo empty vector vs. vector with full-length plastin 3 cDNA construct, pCI-neo/Plastin 3, labeled with Cy3 as described in Materials and Methods appeared as red fluorescence that confirmed successful transfection) was also confirmed by immunofluorescence microscopy, illustrating the fluorescence intensity of plastin 3 (green fluorescence) was induced in pCI-neo/Plastin 3 vs. pCI-neo empty vector which served as a control. Scale bar, 20 µm. Each bar graph in the histograms shown below is a mean ± SD of n = 3 experiments. To assess the intensity of fluorescence signaling of plastin 3, about 25 Sertoli cells were randomly selected from each experiment with n = 3 experiments. (D) Overexpression of plastin 3 was found to perturb the Sertoli cell TJ-permeability barrier. Each data point is a mean ± SD of quadruplicate bicameral units of a representative experiment from n = 3 experiments which yielded similar results. *, P< 0.05.
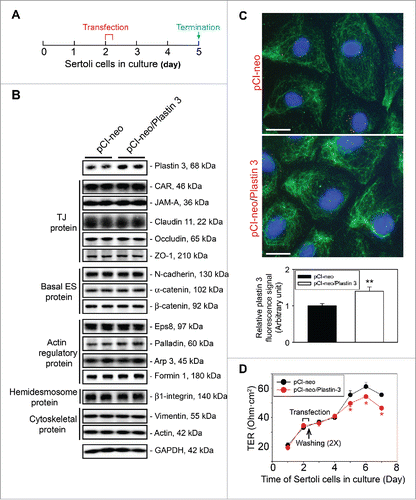
Table 1. Antibodies used for different experiments in this report.
Figure 2. Overexpression of plastin 3 in Sertoli cell epithelium impedes the localization of TJ and basal ES proteins at the cell-cell interface. (A) Immunofluorescence microscopy confirmed overexpression of plastin 3 in Sertoli cells when cells were harvested to assess changes in the plastin 3 (green fluorescence) signals by Image J as described in Materials and Methods. Furthermore, the localization of TJ proteins claudin 11 and ZO-1 and basal ES proteins N-cadherin and ß-catenin was found to be impeded, in which they either rapidly internalized, reducing their levels at the cell-cell interface (e.g., claudin 11 and ZO-1; see white rectangle boxes in control vs. yellow rectangle boxes in plastin 3 overexpressed cells) or no longer tightly but diffusely localized at the cell-cell interface (e.g., N-cadherin and ß-catenin; see white brackets in control vs. yellow brackets in plastin 3 overexpressed cells). These changes thus destabilized the Sertoli cell TJ-permeability barrier as noted in . Scale bar, 20 µm, which applies to all micrographs shown here. These images are representative findings of an experiment from n = 3 independent experiments using different batches of Sertoli cells which yielded similar results. (B) Histograms are either fluorescence signals of plastin 3 across the Sertoli cell (to confirm overexpression of plastin 3), changes in fluorescence signals of claudin 11 and ZO-1 at cell-cell interface between adjacent Sertoli cells (see white or yellow rectangle boxes with signals from 2 opposite ends were scored), or changes in localization of N-cadherin and ß-catenin at cell-cell interface between adjacent cells (bracketed in white or yellow with signals from 2 opposite ends were scored in control (pCI-neo) vs. plastin 3 overexpressed (pCI-neo/Plastin 3) Sertoli cells. Each bar is a mean ± SD of 25 randomly selected Sertoli cells from each experiment with n = 3 experiments were scored (i.e., a total of ∼70 Sertoli cells). **, P < 0.01.
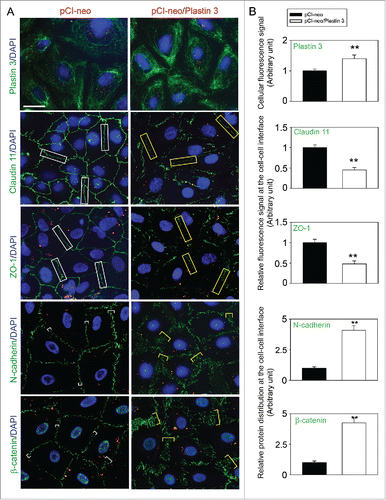
Figure 3. Overexpression of plastin 3 in Sertoli cell epithelium that induces the overall intrinsic actin bundling activity leads to disorganization of actin microfilaments through changes in the spatial expression of Arp3 and Eps8. (A) Overexpression of plastin 3 in Sertoli cells was found to cause dis-organization of actin microfilaments in Sertoli cells in which actin microfilament no longer stretched all across the cell cytosol evenly, instead, they were shown to have actin filaments being bundled at or near the cell cortical zone. These changes appeared to be modulated via changes in the spatial expression of Arp3 (a branched actin-inducing protein) and Eps8 (an actin barbed end capping and bundling protein). However, these changes offset the balance of bundled vs. unbundled/branched actin microfilaments, failing to support F-actin plasticity in the Sertoli cell microenvironment, thereby causing a disruption of the Sertoli cell TJ-permeability barrier as noted in . Scale bar, 20 µm, which applies to all other micrographs. Data shown herein are representative micrographs from a single experiment of n = 3 independent experiments which yielded similar results. (B) Biochemical actin bundling assay using lysates of Sertoli cells overexpressed with plastin 3 (pCI-neo/Plastin 3) vs. control (pCI-neo vector alone) also illustrated an increase in intrinsic actin bundling activity following overexpression of plastin 3 detected in the pellet containing bundled microfilaments vs. supernatant (S/N) containing free microfilaments (see Materials and Methods for details). These findings are representative data of a single experiment with n = 3 independent experiment which yielded similar results as shown in the bar graph on the right panel. Each bar is a mean ± SD of n = 3 independent assays. **, P< 0.01.
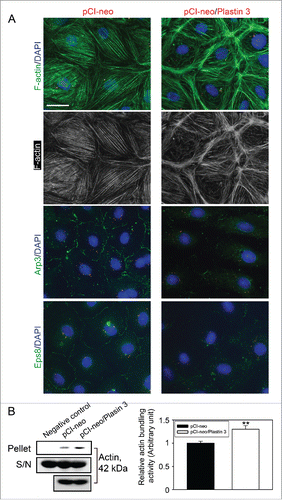
Table 2. Primers used for cloning of the full-length rat plastin 3 cDNA.
