Figures & data
Table 1. Maternal adverse events reported following prenatal tetanus-diphtheria-acellular pertussis (Tdap5) vaccination*
Table 2. Complications of pregnancy, labor, delivery and puerperium reported following prenatal tetanus-diphtheria-acellular pertussis (Tdap5) vaccination
Figure 3. Pregnancy birth outcomes following prenatal tetanus-diphtheria-acellular pertussis (Tdap5) vaccination; Legend: blue = Ectopic Pregnancy, red = Stillbirth/Death in Utero, green = Spontaneous Abortion, and purple = Live Birth.
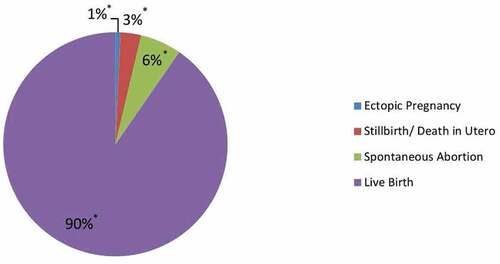
Table 3. Neonatal outcomes following prenatal tetanus-diphtheria-acellular pertussis (Tdap5) vaccination
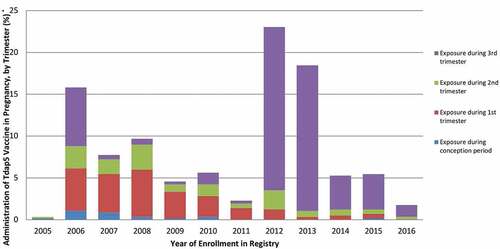
Figure 4. Prenatal Tdap5 vaccination by year and trimester of exposure; Legend: purple = Exposure during 3rd trimester, green = Exposure during 2nd trimester, red = Exposure during 1st trimester, and blue = Exposure during conception period.