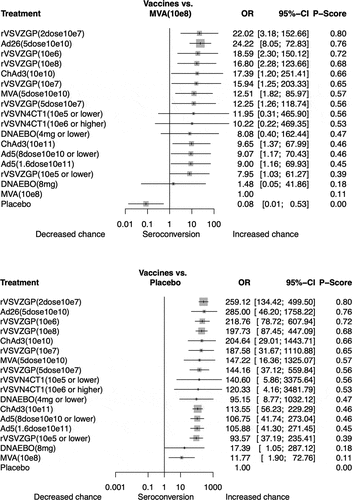
Figures & data
Table 1. Characteristics of included trials investigating the immunogenicity and safety of Ebola virus vaccination
Figure 2. Network graph of eligible Ebola vaccines comparisons for immunogenicity
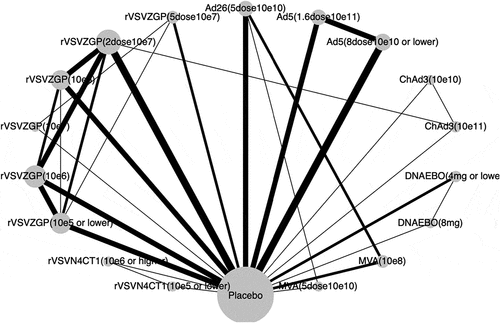
Figure 3. Summary of risk bias assessment for RCTs Ebola vaccines comparisons
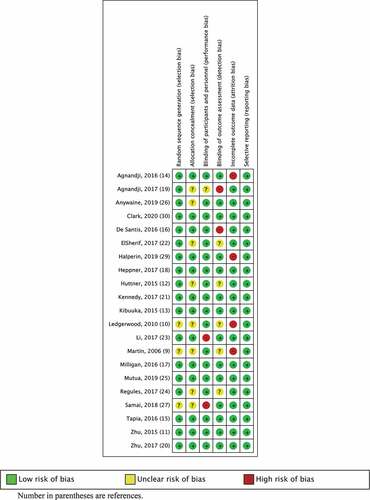
Figure 5. Pairwise comparisons in network meta-analysis for safety outcomes (injection site-pain, fatigue, headache, myalgia, and fever)

Supplemental Material
Download Zip (103.8 MB)Availability of data and materials
The data are available upon request ([email protected]).