Figures & data
Table 1. Characteristics of AEFI reports following human papillomavirus quadrivalent vaccine (4vHPV) from 2018–2020 (N = 238)
Table 2. Serious AEFI and non-serious AEFI reports following human papillomavirus quadrivalent vaccine (4vHPV) from 2018–2020, by city
Figure 1. Reporting rate of adverse event following immunization of 4vHPV from 2018 to 2020, Zhejiang province (/10000 doses).
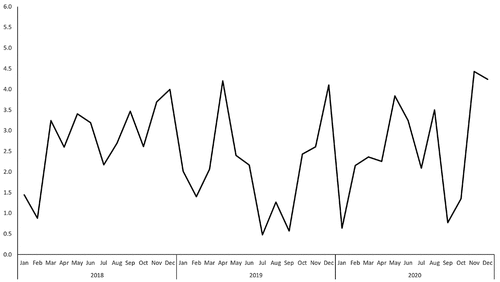
Table 3. Clinical diagnosis of AEFI reports following human papillomavirus quadrivalent vaccine (4vHPV) from 2018–2020
