Figures & data
Table 1. Demographic, occupational, and serological characteristics of the study population
Figure 1. a) Anti-SARS-CoV-2 spike antibody levels after one and two doses of the BNT162b2 mRNA COVID-19 vaccine in SARS-CoV-2-experienced and SARS-CoV-2-naive HCWs. b) Anti-SARS-CoV-2 spike antibody levels after two doses of vaccine in SARS-CoV-2-experienced HCWs who had been diagnosed with SARS-CoV-2 infection for less than 6 months and those who had been diagnosed for more than 6 months at the time of vaccination. Horizontal bars express the median values.
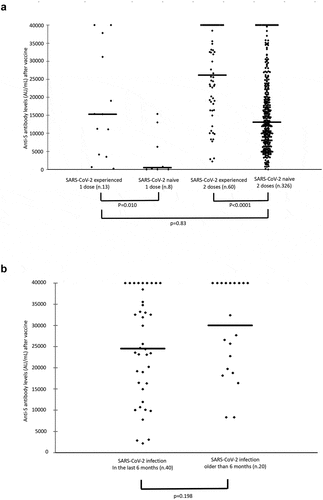
Table 2. Frequency of HCWs reporting vaccine-associated side effects according to SARS-CoV-2 infection status
Figure 2. Relative frequency of vaccine-associated side effects (grade 3) compared between SARS-CoV-2-experienced and -naive participants, between male and females and between HCWs with SARS-CoV-2 infection within the 6 months preceding vaccination and HCWs with a SARS-CoV-2 infection past this time.
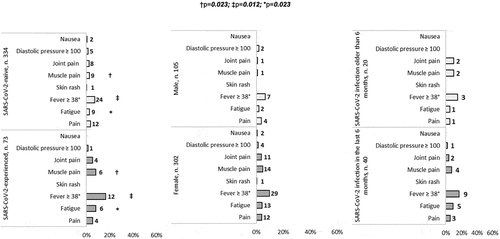
Table 3. Multivariable logistic regression analysis of factors associated with the risk of developing fever ≥38°C, intense myalgias and fatigue after COVID-19 vaccination.*
