Figures & data
Figure 2. Neutralizing antibody titer of anti G1 (ZTR-68) strain induced by the vaccine at 14 days after second immunization. Serum with different dilutions and 1000 PFU rotavirus were mixed with each other at 37°C for 2 h, and then the mixtures were transferred to the 6-well plate covered with a confluent monolayer of MA104 cells and were cultured for 16 h for immunofluorescence. (a) 1000PFU ZTR-68; (b) 1000PFU ZTR-68 and 1:8 diluted serum; (c) 1000PFU ZTR-68 and 1:16 diluted serum; (d) 1000PFU ZTR-68 and 1:32 diluted serum; (e) 1000PFU ZTR-68 and 1:64 diluted serum; (f) 1000PFU ZTR-68 and 1:128 diluted serum; (g) 1000PFU ZTR-68 and 1:256 diluted serum; (h) 1000PFU ZTR-68 and 1:512 diluted serum; (i) cell control. Magnification×10. Bar, 100 μm.
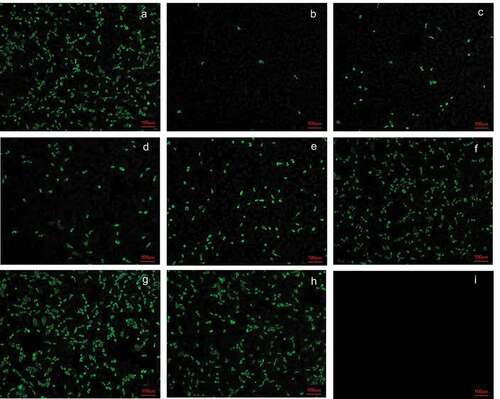
Figure 3. Neutralization antibody titers of antiserum induced by the vaccine. (a) Neutralizing antibody titer of anti G1 (ZTR-68) strain; (b) neutralizing antibody titer of anti G9 (ZTR-18) strain. Data are expressed as the mean ± SD, n = 3, *P ≤ 0.05, **P ≤ 0.01.
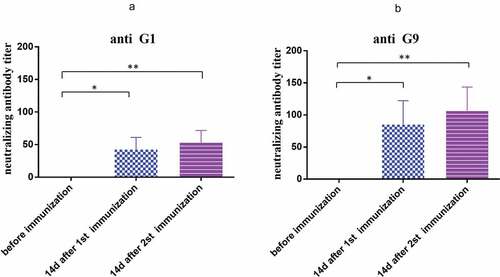
Figure 4. Cross-neutralization antibody titer of anti SA11 strain induced by the vaccine at 14 days after second immunization. Serum with different dilutions and 1000 PFU rotavirus were mixed with each other at 37°C for 2 h, and then the mixtures were transferred to the 6-well plate covered with a confluent monolayer of MA104 cells and were cultured for 16 h for immunofluorescence. (a) 1000PFU SA11; (b) 1000PFU SA11 and 1:8 diluted serum; (c) 1000PFU SA11 and 1:16 diluted serum; (d) 1000PFU SA11 and 1:32 diluted serum; (e) 1000PFU SA11 and 1:64 diluted serum; (f) 1000PFU SA11 and 1:128 diluted serum; (g) 1000PFU SA11 and 1:256 diluted serum; (h) 1000PFU SA11 and 1:512 diluted serum; (i) cell control. Magnification×10. Bar, 100 μm.
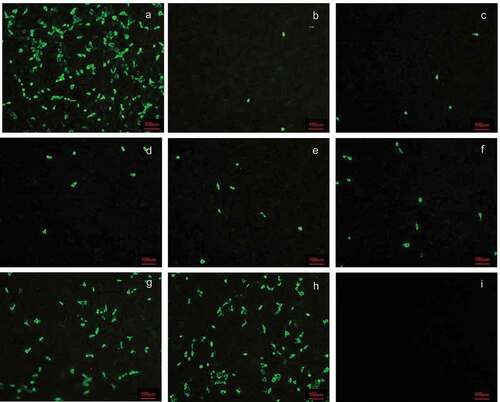
Figure 5. Cross-neutralization antibody titers of antiserum induced by the vaccine. (a) Anti SA11 strain; (b) anti WA strain; (c) anti UK strain; (d) anti Gottfried strain. Data are expressed as the mean ± SD, n = 3, *P ≤ 0.05, **P ≤ 0.01.
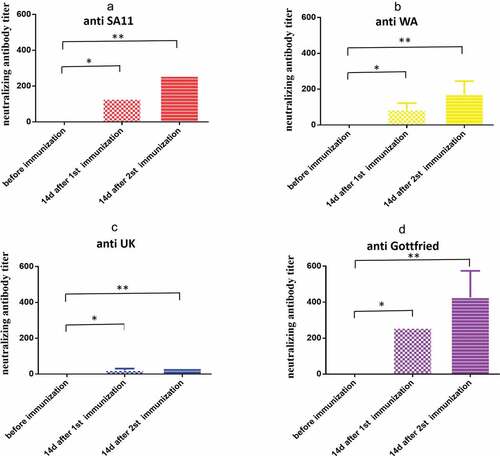
Figure 6. IgA antibody titers in milk in pregnant rhesus monkeys and neutralizing antibody in newborn rhesus monkeys. (a) IgA antibody titers in milk induced by the vaccine (80EU G1 and 160EU G9) or placebo (0.8 mg/ml Al(OH)3 adjuvant) in pregnant rhesus monkeys at 14 days after parturition; (b) maternal-specific rotavirus cross-neutralization antibody titers in newborn rhesus monkeys at 14 days after childbirth. Data are expressed as the mean ± SD, n = 3, *P ≤ 0.05, **P ≤ 0.01.
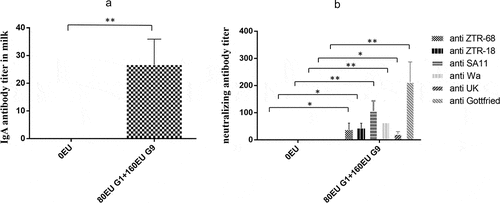
Table 1. Diarrhea score of the immunized neonatal rhesus monkeys from 1 dpi to 3 dpi
Figure 7. Preliminary analysis of immunoprotection in neonatal rhesus monkeys. The neonatal rhesus monkey’ mothers were immunized with 80EU G1 and 160EU G9 inactivated vaccine or placebo (0.8 mg/ml Al(OH)3 adjuvant). (a) The RV antigen-shedding in feces of neonatal rhesus monkeys inoculated with 108PFU SA11 from 0dpi to 3dpi; (b) histopathological changes in the jejunum of neonatal rhesus monkeys infected with 108PFU SA11 at 3dpi in the control group; (c) histopathological changes in the jejunum of neonatal rhesus monkeys infected with 108PFU SA11 at 3dpi in the vaccine group. Magnification ×10. Bar, 100 μm.
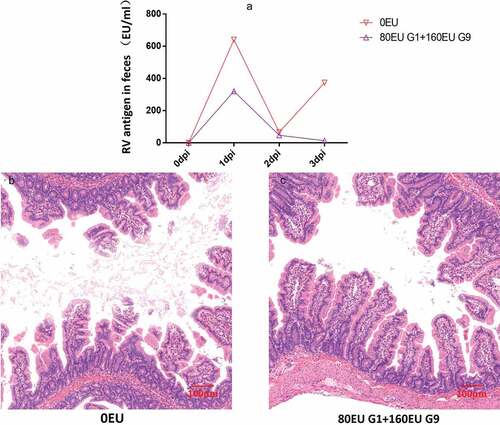
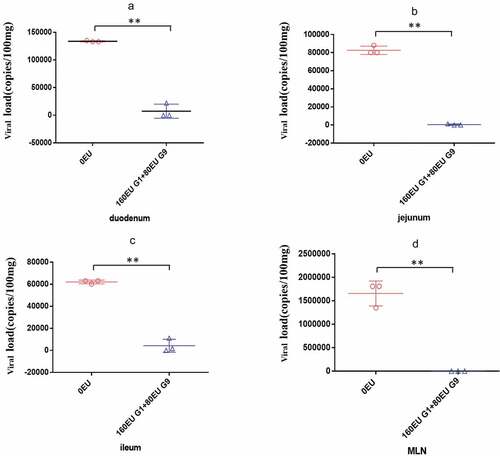
Figure 8. Comparison of viral load in different organs of neonatal rhesus monkeys inoculated with SA11 in vaccine group and control group. (a) Viral load in the duodenum of neonatal rhesus monkeys inoculated with SA11 at 3 dpi; (b) viral load in the jejunum of neonatal rhesus monkeys inoculated with SA11 at 3 dpi; (c) viral load in the ileum of neonatal rhesus monkeys inoculated with SA11 at 3 dpi; (d) viral load in the MLN of neonatal rhesus monkeys inoculated with SA11 at 3 dpi. Data are expressed as the mean ± SD, n = 3, **P ≤ 0.01.