Figures & data
Figure 1. Flowchart of the participant enrolment. ALT, alanine aminotransferase; AST, aspartate aminotransferase; DR, digital radiography; HCV, hepatitis C virus; QFT, QuantiFERON-TB Gold In-Tube; RCT, randomized controlled trial; TB, tuberculosis; ULN, upper limit of normal; WBC, white blood cell. Of the 44,500 recruited rural residents, 43,670 participants aged 18–75 years had complete results of chest radiography and questionnaire, 40,682 were excluded because of normal chest radiographic findings (n = 40,451), suspect TB (n = 119) and other pulmonary diseases (n = 112). The rest 2,988 participants were identified with radiographically inactive TB lesions. Among them, 855 individuals were identified to be QFT-positive and 830 signed the informed consent form for the RCT. Finally, 677 eligible participants were included in the RCT and were randomly classified into two groups: 345 in the preventive treatment group and 332 in the control group without treatment.
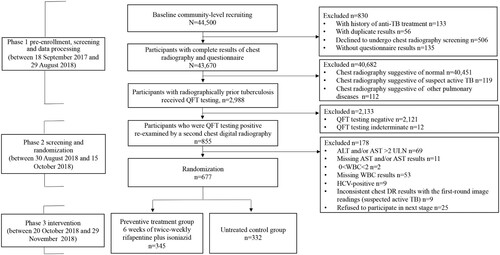
Table 1. Characteristics of the study participants included in the intention-to-treat analysis.
Table 2. Completion of the treatment regimens and attributions for treatment discontinuation.
Table 3. Incidence of active tuberculosis in the study groups.
Table 4. Characteristics of the participants with adverse events and study drug side-effects.
Unmarked_up_Supplementary_materials_R1.docx
Download MS Word (99.4 KB)Data availability statement
This study is registered at www.chictr.org.cn with identifier ChiCTR-1800018224. The corresponding author can provide, upon request, individual participant data that underlie the results reported in this article after applying necessary measures to guarantee that no individual is identified or identifiable.
