Figures & data
Figure 1. Strategies for the development of animal models with susceptibility to human pathogens. Animal models for human infectious diseases imitate clinically affected patients and reflect the processes of pathogen invasion, replication and shedding as well as the innate and adaptive immune response, tissue injuries and lesions, and clinical symptoms. It is essential to clarify the pathogenesis and develop prophylactic and therapeutic strategies against infection and transmission. The screening of animals that exhibit characteristics similar to those of humans in terms of susceptibility to pathogens is essential and constitutes the first step toward establishing animal models for infectious diseases; four strategies for the development of susceptible animal models were reviewed. I. Expression of human viral receptors via transgenic techniques is a common method for enhancing the susceptibility of rodents to human-origin viruses, which can be achieved by inserting the human receptor gene randomly or in a specific site of the mouse chromosome, and then enhancing the mouse cell susceptibility to human viruses. This method facilitates the development of a viral receptor humanized mouse model for SARS-CoV-2, MERS-CoV and EV71. II. The transplantation of human stem cells or tissues into immunocompromised mice and the generation of immune or liver tissue-humanized animals allows infection by strictly human-tropism-related pathogens such as HIV, HBV and HCV. Furthermore, dual-humanized mice with both hepatocytes and immune cells of human origin could reproduce the HBV or HCV lifecycle in vivo and simulate liver pathogenesis processes of chronic hepatitis B patients, such as inflammation, fibrosis, and ultimately cirrhosis. III. The destruction of immune defenses in immunocompetent animals by genetic mutation or deletion of targeted genes can facilitate pathogenic infection. These animals include the severe immunodeficiency inbred strains Nude, SCID, and NOD/SCID and the genetically modified strains with deletions in antiviral restriction factors related to ISGs or signalling cascade pathways of innate immunity. IV. Some animal species are naturally susceptible to human pathogens; for example, ferrets are susceptible to influenza A virus, marmosets are susceptible to MERS-CoV, and hamsters are susceptible to SARS-CoV-2. In addition, recombinant inbred collaborative cross (CC) mice simulate the diversity of the genetic background and susceptibility to pathogens. Therefore, these mice could be used to identify specific lines susceptible to human pathogen infection.
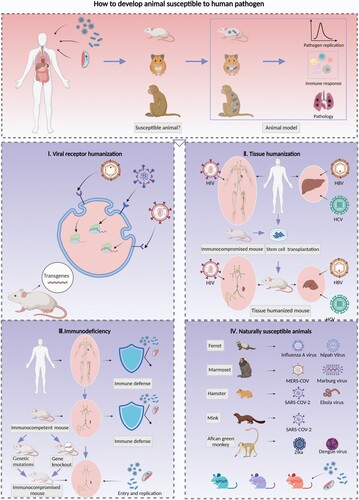
Table 1. Receptors that potentially enhance the susceptibility of animals to human viruses.
Table 2. Immunodeficient animals that are more susceptible to human pathogens.
Table 3. Specific animals that are susceptible to human pathogens.
