Figures & data
Figure 1. Current workflow for carbapenemase testing carbapenem-resistant P. aeruginosa isolates. Automated antimicrobial susceptibility testing was performed with BD Phoenix Platform. CPO well = the detection test for carbapenemase-producing organisms on the BD Phoenix panel. CRE = Enterobacterales resistant to either meropenem or ertapenem. CarbaR, PHL = public health laboratory.
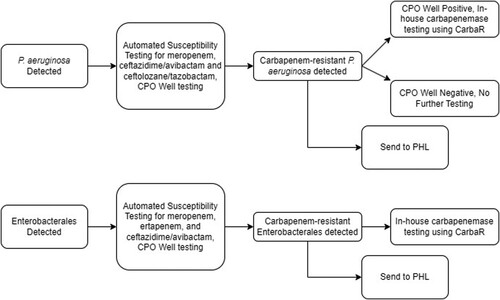
Figure 2. Standard Pathway PHL testing results in a median 6-day delay in receipt of carbapenemase testing results compared with same day results for in-house testing. This delays removal of patients from contact precautions while testing for carbapenemases is being conducted. The 6-day delay negatively impacts rapid therapeutic decisions especially if laboratories have implemented cascade testing. Same day carbapenemase testing can inform treatment decisions if cascade phenotypic susceptibility testing protocols are utilized, resulting in only a ∼24 h delay[Citation19]. Supplemental phenotypic algorithms specifically to identify P. aeruginosa isolates that likely harbour carbapenemases may streamline testing in either scenario.
![Figure 2. Standard Pathway PHL testing results in a median 6-day delay in receipt of carbapenemase testing results compared with same day results for in-house testing. This delays removal of patients from contact precautions while testing for carbapenemases is being conducted. The 6-day delay negatively impacts rapid therapeutic decisions especially if laboratories have implemented cascade testing. Same day carbapenemase testing can inform treatment decisions if cascade phenotypic susceptibility testing protocols are utilized, resulting in only a ∼24 h delay[Citation19]. Supplemental phenotypic algorithms specifically to identify P. aeruginosa isolates that likely harbour carbapenemases may streamline testing in either scenario.](/cms/asset/331ed397-542e-49bb-8457-7dbc030df366/temi_a_2179344_f0002_ob.jpg)
