Figures & data
Figure 1. Study profile. HE: Hepatitis E. HPV: Human papillomavirus. All the women who received at least one dose of vaccine were followed up to 66 months. *By mistake, one participant in the HPV vaccine group was given the HE vaccine for dose 1, and two participants in the HE vaccine group were given the HPV vaccine for dose 3. These three participants were included in the HPV vaccine group for safety analysis, according to the protocol. †One woman with one pregnancy event in the HPV vaccine group was excluded from the analysis due to loss of follow-up and incomplete information on pregnancy outcome. ‡Proximal exposure was defined as vaccination during pregnancy or the onset of pregnancy within 90 days post any dose. Abbreviations: HE, hepatitis E; HPV, human papillomavirus.
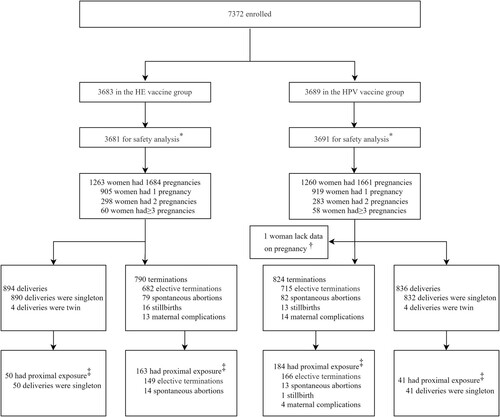
Table 1. Characteristics of HE or HPV vaccinees who became pregnant throughout the study.
Table 2. Overall summary of pregnancy outcomes and complications.
Table 3. Adverse events in women who were inadvertently vaccinated during pregnancy.
Table 4. Association between exposure to vaccination and adverse pregnancy outcomes or pregnancy complications*.

