Figures & data
Figure 1. Flow chart for enrolment, randomization and follow-up of patients. One patient on the Parabolic Response Surface (PRS) regimen permanently changed assigned regimen due to shortage of clofazimine, while the rest were due to adverse drug reactions. NTM: nontuberculous mycobacteria.
Table 1. Baseline characteristics of patients in the modified intention-to-treat population.
Table 2. Primary efficacy analysis in the modified intention-to-treat and per-protocol populations.
Figure 2. Kaplan–Meier analysis of time to culture conversion in the modified intention-to-treat population (A) (n = 93) and per-protocol population (B) (n = 59). The risk table showed the number and percentage of patients at risk of a positive sputum culture at the beginning of each month. No significant differences were observed between patients on Parabolic Response Surface (PRS) or standard regimen (P = .928 for modified intention-to-treat population; P = .298 for per-protocol population).
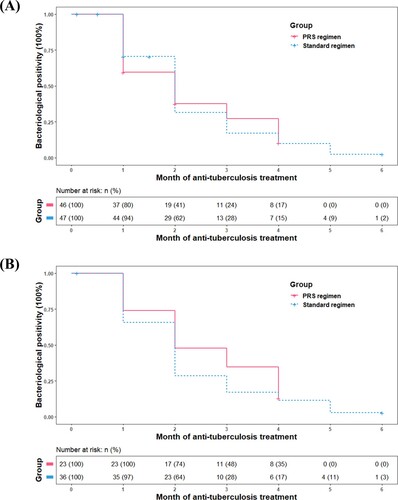
Table 3. Secondary efficacy analysis in the modified intention-to-treat and per-protocol populations.
Figure 3. Early bactericidal activity (EBA) of the standard and Parabolic Response Surface (PRS) regimens in the modified intention-to-treat population with assessable results. The number of sputum samples tested at each time point are presented in the figures, after excluding the missing (mainly due to the difficulty of expectorating), contaminated or low-quality sputum samples. No significant between-group differences were identified on EBA0-2 days (median: 0.27 vs. 0.46 log10 CFU ml−1 d−1, P = .177), EBA2-14 days (0.19 vs. 0.10 log10 CFU ml−1 d−1, P = .182), nor EBA0-14 days (0.19 vs. 0.22 log10 CFU ml−1 d−1, P = .739).
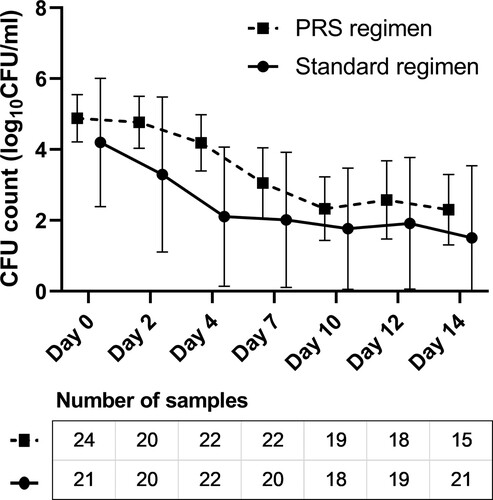
Table 4. Safety analysis in all randomized patients receiving at least one dose of the trial medication.
