Figures & data
Figure 1. Recombinant adenovirus constructs and in vitro protein expression. (a) Schematic representation of the recombinant adenovirus (rAd) constructs. All the constructs were designed to express the HA stem domain, which contains the tyrosinase signaling peptide (SP) at the N-terminus of HA1-stem. (b) In vitro protein expression in mouse cell line. TC-1 mouse lung cells were infected with rAds and cell lysates were collected. Protein expression was confirmed using anti-HA2 polyclonal and anti-RSV-F (palivizumab) monoclonal antibodies. The line arrows indicate the HAstem and HAstem-RSVT-cell protein bands detected by the anti-HA2 antibody.
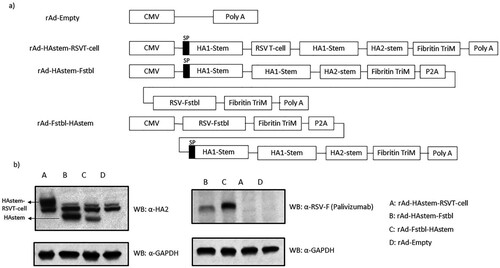
Table 1. T-cell epitope sequences included in the design of rAd-HAstem-RSVTcell.
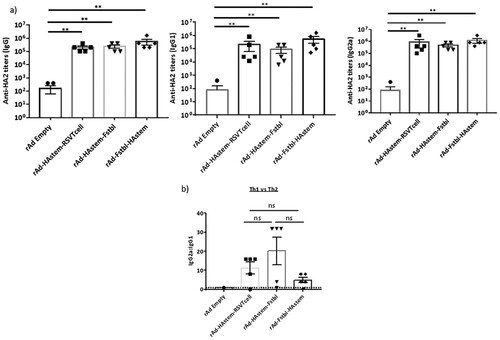
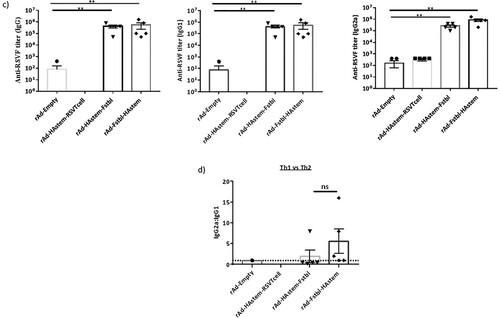
Figure 3. Serum antibodies possess neutralizing and ADCC effector functions against influenza and RSV. (a,b) Serum collected post-boosting was used to determine the neutralizing ability (a) and ADCC activity (b) against influenza A/Netherlands/602/09 (H1N1). Anti-Influenza A/California/7/09 HA serum (NIBSC code – 14/310) was used as a positive control for the neutralization assay. RSV-A2 neutralizing ability (c) and ADCC activity against RSV-A2 (d) of the mice serum collected post-boosting (n = 5) is shown. (b,d) Fold induction over “no antibody” control is shown. Data shown is mean ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001. (two-wayANOVA with Tukey's post hoc test (b), paired t-test (c), Dunnett's post hoc test (d)).
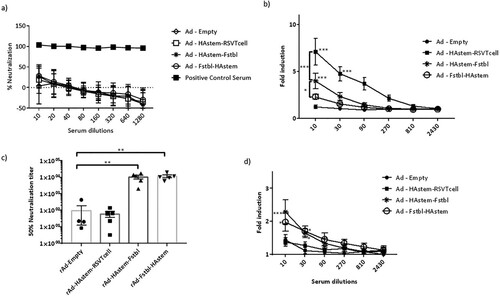
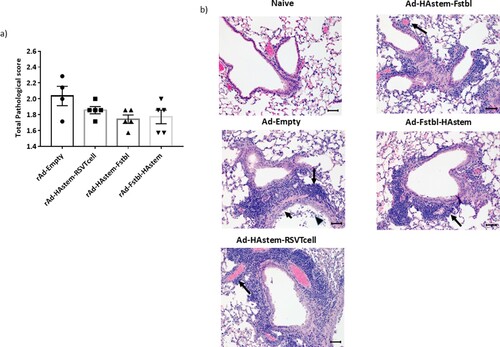
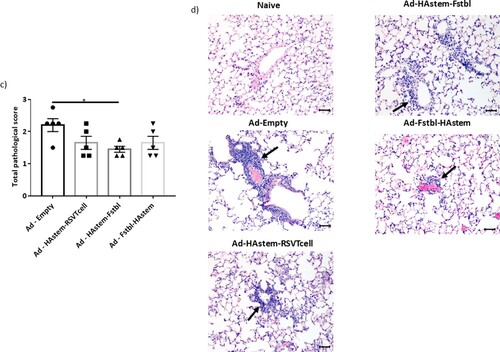
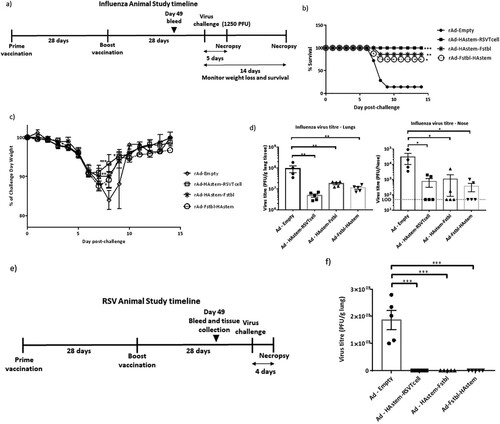
Figure 5. Intranasal immunization with the vaccine constructs provide significant protection against influenza and RSV challenge. (a) Schematic diagram of the immunization, influenza A challenge and necropsy timeline at a challenge dose of 1250 PFU/mouse. (b–d) BALB/c mice were vaccinated intranasally in a prime/boost regimen, 4-weeks apart, with the rAd vaccines and challenged intranasally with 1250 PFU/mouse of influenza A. (b) Survival (n = 7 or 8) is shown. Median survival time since day of challenge – Ad-empty: 7 days, all vaccinated groups: > 14 days. *p < 0.05, **p < 0.01, ***p < 0.001, log-rank test. (c) Weight loss data is shown. Data shown is mean ± SEM. (d) Virus titre from infected lungs and nose collected 5 days post-challenge were determine by plaque assay (n = 4 or 5). Data shown is mean ± SEM. The dotted line denotes the limit of detection (LOD) of the assay. *p < 0.05, **p < 0.01, ***p < 0.001 (two-way ANOVA (Tukey's post hoc test) (c), one-way ANOVA (Dunnet's post hoc test) for lung viral titers and Kruskal-Wallis (non-parametric) test for the Nose viral titers (d). (e) Schematic diagram of the immunization, RSV-A2 challenge and necropsy timeline. (f) BALB/c mice were vaccinated intranasally in a prime/boost regimen, 4-weeks apart, with the rAd vaccines and challenged intranasally with RSV-A2. Virus titre from infected lungs collected 4 days post-challenge were determine by plaque assay (n = 5). Data shown is mean ± SEM. ***p < 0.001.one-way ANOVA (Dunnet's post hoc test).
Supplemental Material
Download MS Power Point (305.1 KB)Data availability statement
Data have been deposited in the Government of Canada Centralized repertoire and would be available upon request after publications.