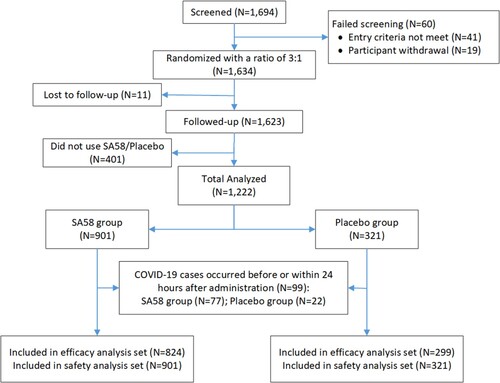
Figures & data
Table 1. Baseline characteristics of study participants (full analysis set).
Figure 2. Time to first post-administration symptomatic COVID-19 (i.e. the Kaplan–Meier estimates of the cumulative risk of having COVID-19). Panel A. laboratory-confirmed symptomatic COVID-19; Panel B. SARS-CoV-2 RT-PCR positive. Abbreviation, HR, Hazard ratio; 95%CI, 95% confidence interval.
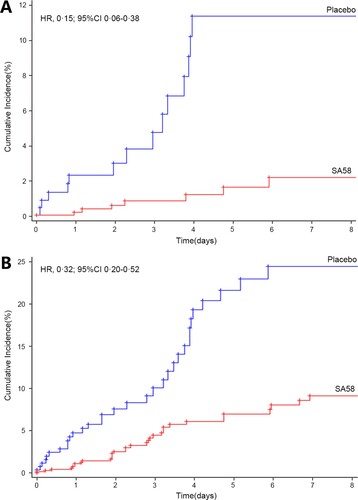
Table 2. Occurrence of SARS-CoV-2 RT-PCR positive and laboratory-confirmed symptomatic COVID-19 in SA58- and placebo-treated participants.
Table 3. Summary of adverse events.