Figures & data
Table 1. Mortality, virus shedding, and seroconversion of chickens inoculated with and contact of clade 2.3.4.4 highly pathogenic avian influenza viruses. Inoculum dose for intrachoanal inoculation included in first column.
Figure 1. Egg infectious dose-equivalent titers from the oropharyngeal (OP) and cloacal (CL) swabs collected from chickens inoculated with or in contact with chickens shedding clade 2.3.4.4 highly pathogenic avian influenza viruses. Chickens in contact group were transferred into the isolators with the inoculated birds on 1 dpi. All samples were negative at 7, 10, and 14 dpi and are not shown. The lower detection limit was 102.5 50% egg infectious dose (EID50)/mL. Negative RT-PCR samples were treated as 102.4 EID50/mL for statistical purposes.
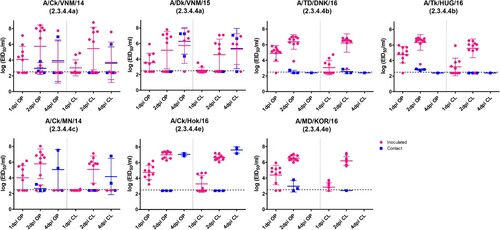
Figure 2. Histopathology and immunohistochemical staining for AIV antigen in tissues of chickens infected with clade 2.3.4.4 highly pathogenic avian influenza viruses, 2 days post-inoculation. Hematoxylin-eosin staining (A-E); immunohistochemistry (insets), virus staining of cells (nuclei – clearly stained circular bodies) in red. A. Lung, A/Ck/VNM/14 (clade 2.3.4.4a). Interstitial pneumonia with lymphoplasmacytic infiltrates. Inset: Lung, same area. Viral antigen in endothelial cells, pneumocytes, mononuclear cells. B. Pancreas, A/TD/DNK/16 (clade 2.3.4.4b). Necrotic pancreatic acinar cells with lymphoplasmacytic infiltrates. Inset: Pancreas, same area. Viral antigen in acinar cells, duct cells, endothelial cells. C. Thymus, A/TD/DNK/16 (clade 2.3.4.4b). Necrosis in medulla and lymphocyte depletion in cortex. Inset: Thymus, same area. Viral antigen in mononuclear cells, thymic epithelium in medullary area, endothelial cells. D. Duodenum, A/Ck/Hok/16 (clade 2.3.4.4e). Focal necrosis with lymphoplasmacytic infiltrates in submucosa. Inset: Duodenum, same area. Viral antigen in enterocytes, endothelial cells, mononuclear cells in lymphoid associated tissue, smooth muscle cells. E. Cecal tonsil, A/MD/KOR/16 (clade 2.3.4.4e). Focal necrosis with lymphoplasmacytic infiltrates in submucosa. Inset: Cecal tonsil, same area. Viral antigen in enterocytes, endothelial cells, mononuclear cells in lymphoid associated tissue.
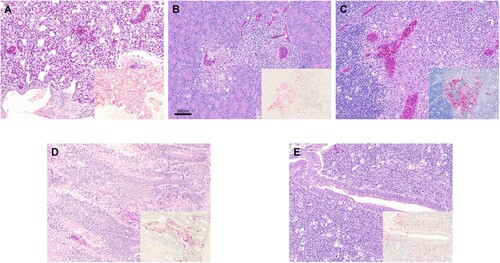
Table 2. Microscopic lesions and viral antigen distribution in chickens inoculated with clade 2.3.4.4 viruses, 2 days post-inoculation.
Table 3. Microscopic lesions and viral antigen distribution in chickens inoculated with clade 2.3.4.4a-e highly pathogenic avian influenza viruses, 2 days post-inoculation.
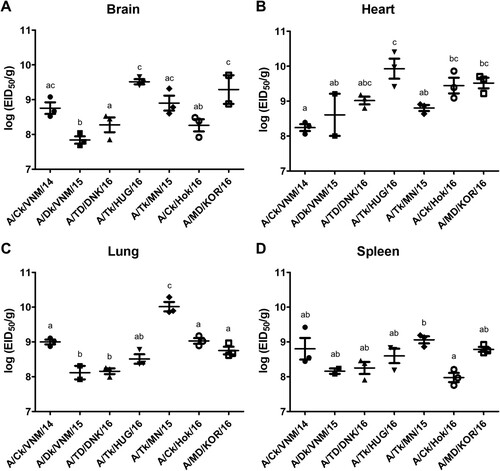
Figure 3. Egg infectious dose-equivalent titers in brain (A), heart (B), lung (C) and spleen (D) collected from chickens inoculated with clade 2.3.4.4 highly pathogenic avian influenza viruses at 2 days post-inoculation (n = 3 chickens/virus). Viral titers sharing at least one superscript letter indicate no statistically significant differences (p ≥ 0.05).