Figures & data
Figure 1. Study design & patient assessment timing. Abbreviations: ACQ-6, Asthma Control Questionnaire, 6 Item; AQLQ, Asthma Quality of Life Questionnaire; EQ-5D-5L, EuroQol 5 Dimension, 5-level; HCRU, Healthcare resource utilization; Med, Medication; PGI-C/S: Patients’ Global Impression of Change/Severity; SAE, Serious adverse event; TSQM-9, Treatment Satisfaction Questionnaire for Medication, Version 9.
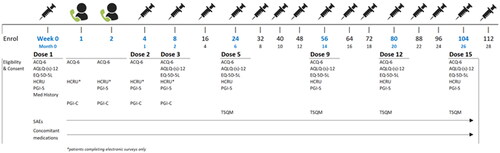
Table 1. Patient demographics in patients with baseline assessments.
Figure 2. Mean asthma control (ACQ-6) score and change in ACQ-6 score from baseline. Abbreviations: ACQ-6, Asthma Control Questionnaire, 6 Item.
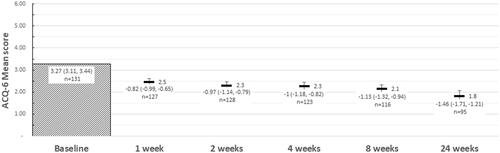
Figure 3. Mean ACQ-6 scores, and change in ACQ-6 scores from baseline at each follow-up, by response at 24 weeks follow-up. Abbreviations: ACQ-6, Asthma Control Questionnaire, 6 Item. At 24 weeks, “Uncontrolled” asthma: ACQ-6 score ≥1.5; “partial control”: ACQ-6 score >0.75 and <1.5; “controlled” asthma: ACQ-6 score ≤0.75.
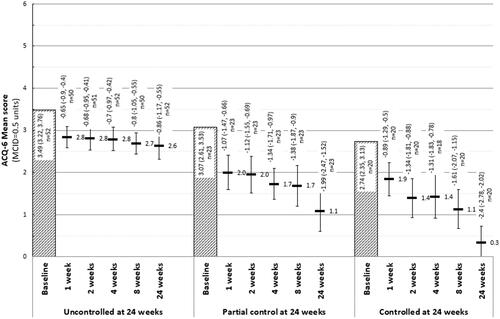
Figure 4. (A) Mean asthma quality of life (AQLQ) scores, and change in AQLQ scores from baseline at each follow-up; (B) General quality of life (EQ-5D-5L utilities) and change from from baseline at each follow-up. Abbreviations: AQLQ, Asthma Quality of Life Questionnaire; EQ-5D-5L, EuroQol 5 Dimension, 5-level.
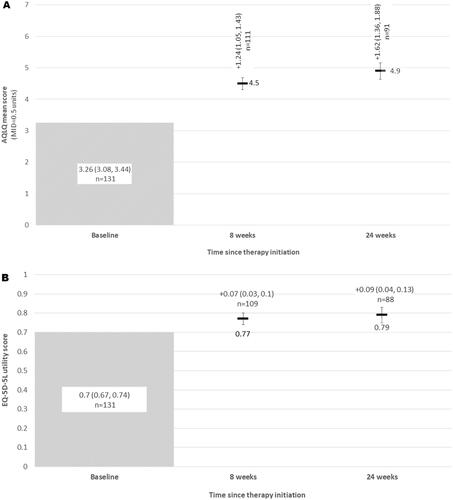
Figure 5. Change from baseline in AQLQ over 24 weeks of treatment, stratified by ACQ-6 responder status at 24 weeks. Abbreviations: ACQ-6, Asthma Control Questionnaire, 6 Item; AQLQ, Asthma Quality of Life Questionnaire. At 24 weeks, “Uncontrolled” asthma: ACQ-6 ≥ 1.5; “partial control”: ACQ-6 > 0.75 and <1.5; “controlled” asthma: ACQ-6 ≤ 0.75.
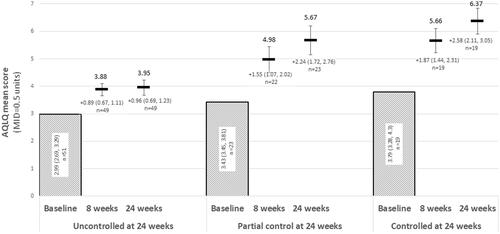
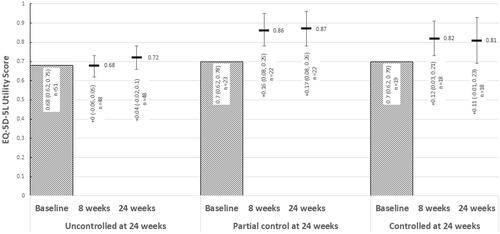
Figure 6. Change from baseline in EQ-5D-5L utilities over 24 weeks of treatment, stratified by ACQ-6 responder status at 24 weeks. Abbreviations: ACQ-6, Asthma Control Questionnaire, 6 Item; EQ-5D-5L, EuroQol 5 Dimension, 5-level. At 24 weeks, “Uncontrolled” asthma: ACQ-6 ≥ 1.5; “partial control”: ACQ-6 > 0.75 and <1.5; “controlled” asthma: ACQ-6 ≤ 0.75.