Figures & data
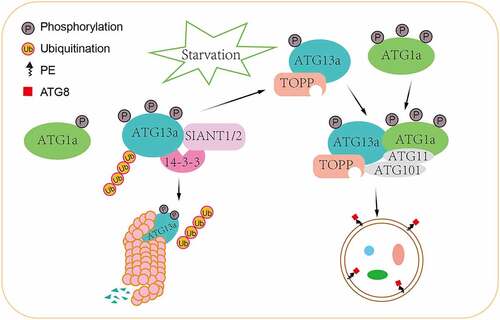
Figure 1. A proposed working model of 14-3-3 proteins in plant autophagy. Under nutrient-rich conditions, 14-3-3 proteins inhibit autophagy by recruiting E3 ubiquitin ligases SINAT1 and SINAT2 to degrade phosphorylated ATG13 via the 26S proteasome pathway and also promote the dissociation of the ATG1-ATG13 complex, thus leading to the suppression of autophagy. Under nutrient-deprivation conditions, the dephosphorylation of ATG13a is mediated by TOPP, leading to reduced affinity with 14-3-3 proteins and increased binding with hyperphosphorylated ATG1a, thus facilitating autophagy initiation events in plants.