Figures & data
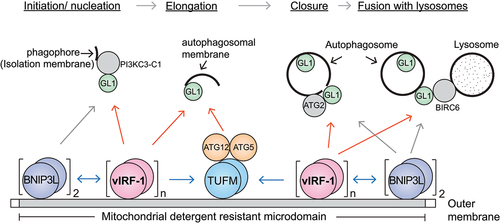
Figure 1. Proposed model of vIRF-1-mediated mitophagy. vIRF-1 is expressed following lytic reactivation and localized to mitochondria by targeting detergent-resistant membrane domains where it interacts with the autophagy proteins BNIP3L, TUFM, and GABARAPL1 (GL1). BNIP3L and vIRF-1 exert on each other for multimerization to be autophagy-competent and may recruit the autophagy machinery to the mitochondria by interacting with GL1. GL1 is known to interact with PI3KC3-C1 (phosphatidylinositol 3-kinase catalytic subunit type 3 complex 1), ATG2, and BIRC6 (baculoviral IAP repeat containing 6). In addition, vIRF-1 may promote autophagosome formation by recruiting ATG12-ATG5 proteins to the mitochondria by promoting the formation of autophagy-competent TUFM (the dimerized form of pre-TUFM).