Figures & data
Figure 1 Common functions of tissue inhibitors of metalloproteinases (TIMPs) in microvascular endothelial cell (MVEC) barrier function.
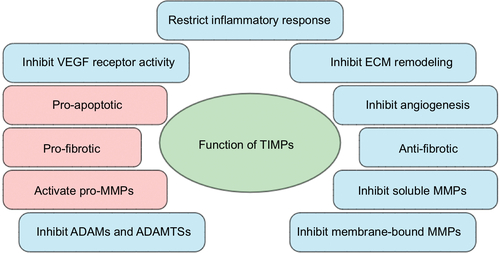
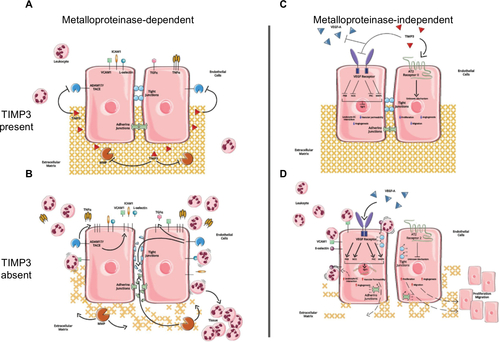
Figure 2 Predicted metalloproteinase-dependent and metalloproteinase-independent functions of TIMP3 in MVECs.
Abbreviations: TIMP3, tissue inhibitors of metalloproteinases; MVECs, microvascular endothelial cells; MMP, matrix metalloproteinases; ECM, extracellular matrix; ADAM, a disintegrin and metalloproteinase; TNFα, tumor necrosis factor alpha.