Figures & data
Table 1 Quality by testing versus quality by design in pharmaceutical development
Table 2 Critical criteria for development of a topical dermatological dosage form
Table 3 General elements of a QTPP for a TDDF
Table 4 Hypothetical risk assessment using failure mode effects and criticality analysis for lack of viscosity and particle size analysis
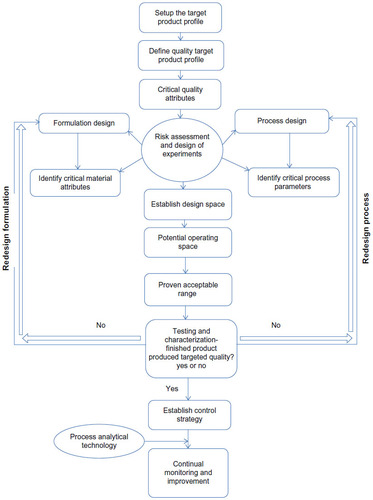
Figure 2 General quality by design approach for development of a topical dermatological dosage form.Citation25
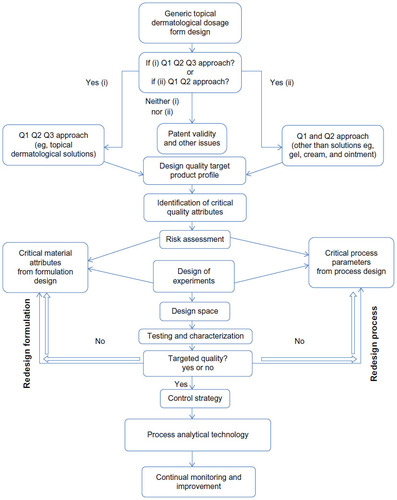
Table 5 Commonly used excipients and their challenges during development of a topical dermatological dosage form
Table 6 Types of levels and descriptions