Abstract
Objective
To evaluate and compare the associations of VEGFA serum levels and SNPs (rs1570360, rs699947, rs3025033, and rs2146323) with periodontitis in study participants grouped by gender.
Methods
The study enrolled 261 patients with periodontitis and 441 healthy controls as a reference group. Patients underwent periodontal examination and radiographic analysis to confirm the periodontitis diagnosis. Blood samples were collected, and the DNA salting-out method was used for DNA extraction from peripheral venous blood. Genotyping of VEGFA (rs1570360, rs699947, rs3025033, and rs2146323) was performed using real-time polymerase chain reaction (RT-PCR) and serum level analysis was done for 80 individuals − 40 periodontitis-affected patients and 40 reference group subjects.
Results
The analysis of VEGFA (rs1570360, rs699947, rs3025033, and rs2146323) showed that the rs3025033 GG genotype was less frequent in the periodontitis group than in the reference group (1.6% vs. 5.7%,p = 0.008). VEGFA serum levels were not statistically significantly different between periodontitis patients and reference group subjects (554.29 (522.38) ng/ml vs. 581.32 (348.16) ng/ml, p = 0.786). Individuals carrying rs1570360, rs699947, rs3025033, and rs2146323 haplotype A-A-G-A had decreased risks of periodontitis, while rare haplotype of VEGFA (rs1570360, rs699947, rs3025033, and rs2146323) was associated with increased odds of periodontitis (OR= 0.42; 95% CI: 0.20–0.85; p < 0.017; OR= 4.08; 95% CI: 1.86–8.94; p < 0.0001, respectively).
Conclusion
The rs3025033 GG genotype and the rs1570360, rs699947, rs3025033, and rs2146323 A-A-G-A haplotypes may play a protective role in the development of periodontitis, but a less common haplotype of the same VEGFA polymorphism may be associated with the risk of developing periodontitis.
Introduction
Periodontitis is the 6th most prevalent chronic disease of mankind, affecting about 10% of the adult global population in the severe form. If untreated, it is a bacterial-driven non-resolving inflammation that leads to tooth loss due to the progressive destruction of the tooth-supporting apparatus with negative effects on chewing function and quality of life [Citation1,Citation2]. Recent advances in geroscience have shown that various biomarker signatures of biological ageing are longitudinally associated with declined physical function, morbidity, and mortality due to major age-related diseases, including periodontitis [Citation3]. Interestingly, periodontitis has a documented higher prevalence in men (∼57%) compared to women (∼39%), signifying a possible sex bias in disease pathogenesis [Citation4]. This disease is responsible for a large proportion of edentulous people and people with masticatory dysfunctions, leads to a significant increase in dental care costs and has a negative impact on overall health [Citation5]. During periodontitis, host defence can lead to the loss of periodontal attachment, alveolar bone and, eventually, of the tooth [Citation6,Citation7].
The periodontal vasculature is profoundly affected during onset and progression of periodontitis. The activity of cytokines and growth factors such as TGF-α, TGF-β, PDGF, FGF, PGE, IL- 1, IL-6 and IL-8 and endotoxins, stimulates the production of VEGF, which is one of the most important proangiogenic factors [Citation8]. It is also known that periodontitis is highly associated with genetic factors and molecular mechanisms [Citation9].
Vascular endothelial growth factor (VEGF), an angiogenic cytokie, is involved in the progression of pathological conditions [Citation10]. There are seven subtypes in the VEGF family: VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, VEGF-F, and PlGF. VEGF-A is the most potent member, with its gene (VEGFA) location on chromosome 6p21.3, and its coding region spans over 14 kb [Citation11]. VEGFA gene consists of eight different exons and is localized in periodontal tissue in endothelial cells, macrophages, and plasma cells, and is also found in gingival crevicular fluid [Citation12]. The functions of VEGFA are diverse: it may be involved in cell adhesion, chemotaxis, cell proliferation, along with regulation of blood vessel development and extracellular matrix remodelling [Citation13]. It also stimulates nitric oxide production and is involved in bone resorption [Citation14].
Periodontal inflammation affects the vasculature of periodontium and enhances the expression of specific mediators, along with the promotion of angiogenesis [Citation15]. VEGFA is involved in both pathological and physiological neovascularization, and its expression is increased in periodontitis patients [Citation16]. Several mediators, such as tumour necrosis factor α, IL-1, IL-6, IL-8, endotoxins, prostaglandin E and fibroblast growth factor, stimulate the synthesis of VEGFA [Citation17]. However, it is important to note that VEGFA levels can increase, decrease, or remain unchanged [Citation18]. It is also important to note, that some researchers report a decrease in VEGF expression after patients undergo periodontal treatment [Citation17,Citation19], although others state that it remains unchanged [Citation20]. This leads to conflicting scientific findings regarding VEGFA expression in periodontitis patients [Citation21]. Differences in reporting whether expression increased or decreased could be caused by various reasons, such as induction of proinflammatory cytokines or periodontopathic pathogens [Citation18,Citation22]. Therefore, we performed a comprehensive analysis to evaluate and confirm the associations of VEGFA with periodontal inflammation; we focused on the intron region (rs1570360 − 1154 G > A (chromosome 6:43770093 (GRCh38)), rs699947 − 2578 C > A (chromosome 6:43768652 (GRCh38)), rs3025033 18123 A > G (chromosome 6:43783338 (GRCh38)), rs2146323 12143 C > A (chromosome 6:43777358 (GRCh38))) as it has been shown to be highly polymorphic and the most studied polymorphisms [Citation23].
This study aimed to evaluate and compare the associations of VEGFA serum levels and SNPs (rs1570360, rs699947, rs3025033, and rs2146323) with periodontitis in study participants grouped by gender.
Materials and methods
Ethical approval and data protection
The study was conducted at the Department of Prosthodontics, Lithuanian University of Health Sciences Hospital, and the Neuroscience Institute of the Lithuanian University of Health Sciences. The Ethics Committee for Biomedical Research approved the study (No. BE −2-20). All subjects gave written informed consent in accordance with the Declaration of Helsinki. Personal data containing sensitive information (first and last name, age, sex, medical history) were coded.
Materials
Inclusion and exclusion criteria
The study was performed on Lithuanian women and men, who came in for a check-up to the periodontist or prosthodontist in Lithuanian University of Health Sciences Hospital, Kaunas Clinics, and fulfilled the inclusion criteria along with an agreement to participate in this analytical cross-sectional study. The timeframe of patients enrolment in the study was from 2021 May to 2022 June. In total there were 261 patients with periodontitis (mean age 70 ± 17 years) and 441 reference patients (mean age 69 ± 18 years). Patients were selected according to the following criteria:
Inclusion criteria for periodontitis affected patients for this study were (in accordance to the ‘2017 Classification of Periodontal and Peri-implant Diseases and Conditions’ classification of periodontal diseases [Citation24] (source: https://aap.onlinelibrary.wiley.com/toc/19433670/2018/89/S1 and https://www.perio.org/wp-content/uploads/2019/08/Staging-and-Grading-Periodontitis.pdf):
Patients age >18-years old;
patient’s informed and voluntary consent to participate in the study, which included radiographic and intraoral periodontal examination to determine the extent of periodontal disease;
generalized periodontitis of stages III and IV - more than 30% of the patient’s oral region affected by periodontitis (examined on their first visit to either the prosthodontist or periodontist
radiographic evidence of bone loss;
interdental clinical attachment loss ≥5mm (III–IVth stage periodontitis);
tooth loss due to periodontitis;
vertical bone loss ≥3mm;
<20 remaining teeth (10 opposing pairs);
Probing depths ≥6mm;
Inclusion criteria for the reference group individuals for this study were:
no bleeding on probing (BOP);
no clinical signs of gingiva inflammation;
no clinical attachment loss was present, and probing depth was ≤3mm;
no previous history of periodontal diseases.
Exclusion criteria for both groups (periodontitis and reference) of this study were:
patients, who were undergoing orthodontic treatment;
diabetes mellitus affected patients;
patients with any medical records of chronic inflammatory diseases, HIV, hepatitis, autoimmune disorders;
pregnant or breastfeeding patients;
patients, who had any type of infection, which required antibiotic treatment in the last 3 months;
patients, under chemotherapy treatment (active or history of chemotherapy).
Intraoral periodontal and radiographic examinations took place during the patient’s first visit to the prosthodontist or periodontist. The diagnosis of periodontal disease was determined according to the consensus report of Working Group 2 of the World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions 2017 [Citation24].
Methods
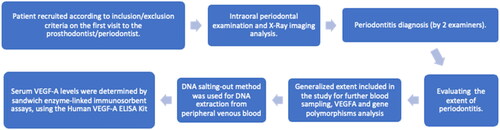
Patients were recruited according to the inclusion and exclusion criteria for this study. Each patient was subjected to clinical periodontal and radiographic examination. Periodontal probing, along with radiographic examinations (orthopantomogram, dental radiographs, and proximal digital radiographs) were performed on the patients affected by periodontitis to evaluate bone loss. Manual probe measurements were carried out using periodontal probe Colorvue (manufacturer Hu-Friedy Mfg. Co., Inc. Zweigniederlassung Deutschland) and bone measurements were made digitally by using calibrated radiographical imaging with digital measurement scale. After examination, patients, affected by any of the four stages of periodontitis (I, II, III and IV) were included in the study. The clinical parameter that divided patients into groups was the extent of the disease. Our study included patients with a generalized distribution of disease (> 30% of the teeth were affected (all four stages of periodontal inflammation were included)).
There were 2 investigators (a prosthodontist and a periodontist) who analyzed both the intraoral examination data and radiographs to conclude the diagnosis of periodontitis and to evaluate the patient for the generalized extent of disease (). Both investigators have used Williams periodontal probes (Michigan O probe, Hu-Friedy Mfg. Co, Chicago, IL USA) to analyze the extent of the disease intraorally by measuring the interdental CAL at site of greatest loss (as it is stated in the ‘2017 Classification of Periodontal and Peri-implant Diseases and Conditions’ [Citation24]). Radiographical analysis was performed, and the measurements were done by the same machine and the same program.
Sample preparation, DNA extraction, genotyping, and enzyme-linked immunosorbent assay
Blood samples were collected by venipuncture, using tourniquet. BD Vacutainer needles and BD Vacutainer® spray-coated K2 EDTA Tubes (for whole blood haematology determinations) and BD Vacutainer™ Glass ACD Solution Tubes (with yellow closure, to obtain whole blood or plasma sample) were used.
Vacuum tubes with EDTA were used to draw a predetermined blood volume for DNA extraction. After collecting the whole blood, it was left undisturbed at room temperature. Usually, it took 15 to 30 min. Then, the clot was removed by centrifuging at 1000–2000 x g for 10 min in a refrigerated centrifuge. In the end, serum was poured into Eppendorf. EDTA (violet colour-coded) tubes were used for DNA and plasma analysis, whereas when the Clot Activator & Gel Tubes (yellow colour-coded tubes) were used for serum analysis.
DNA salting-out method was used for DNA extraction from peripheral venous blood. VEGFA (rs1570360, rs699947, rs3025033, and rs2146323) genotyping was performed using TaqMan®® genotyping assays (Applied Biosystems Foster City, CA, USA) according to the manufacturer’s instructions using real-time polymerase chain reaction (RT-PCR). A serum VEGFA levels were evaluated in duplicates for 40 periodontitis-affected patients and 40 reference group subjects. Both groups consisted of 20 females and 20 males. Samples were randomly selected. Serum VEGF-A levels were determined by sandwich enzyme-linked immunosorbent assays, using the Human VEGF-A ELISA Kit (Cat. No. BMS277- 2TEN) following the manufacturer’s instructions. Results were observed using a Multiskan FC microplate photometer (Thermo Scientific, Waltham, MA, USA) optical density was measured at 450 nm. VEGF-A assay range of the standard curve was: 23.4–1500 pg/mL, and assay analytical sensitivity was <5 pg/mL.
Statistical analysis
Statistical analysis was performed using SPSS/W 29.0 software (Statistical Package for the Social Sciences for Windows, Inc., Chicago, IL, USA). Descriptive data were evaluated for normality using the Kolmogorov–Smirnov test. Continuous variables were presented as median with interquartile range (IQR) or mean and standard deviation based on data distribution. The Mann–Whitney test was used to compare two groups for non-normally distributed data.
Absolute numbers with percentages were used for genotype frequency expressions. The χ2 test was used for the distribution comparison of VEGFA (rs1570360, rs699947, rs3025033, and rs2146323) in the periodontitis and reference groups. Binominal regression analysis was used to estimate the association of genotypes on the presence of periodontitis. Odds ratios (OR) and 95% confidence intervals (CI) are presented. Akaike Information Criterion (AIC) was used for the best genetic model selection; therefore, the best genetic models were represented by the lowest AIC values.
Binary logistic regression results were expressed as statistical genetic models (codominant: heterozygotes vs. major allele homozygotes and minor allele homozygotes vs. major allele homozygotes; dominant: minor allele homozygotes and heterozygotes vs. major allele homozygotes; recessive: minor allele homozygotes vs. major allele homozygotes and heterozygotes; overdominant: heterozygotes vs. homozygotes with major allele and minor allele homozygotes; for the evaluation of the impact of each minor allele of genotype on periodontitis, additive model was used: minor allele homozygotes vs. heterozygotes vs. major allele homozygotes).
SNPStats online software was used to perform haplotype analysis (https://www. snpstats.net/snpstats/(accessed 2 April, 2022) [Citation25]). Haplotype analysis was performed using the online SNPStats program (https://www.snpstats.net/start.htm). D′ (deviation between the expected haplotype frequency and the observed frequency) and r2 (square of the haplotype frequency correlation coefficient) measures were used for the linkage disequilibrium (LD) analysis assessment. Associations of haplotypes with periodontitis were assessed by logistic regression, using OR with a 95% CI. Statistically significant differences were observed when p < 0.05.
After Bonferroni correction, statistically significant differences were observed when p < 0.0125.
Results
Genotype and allele associations with periodontitis
The study enrolled 261 patients with periodontitis: 107 (41.00%) men and 154 (59.0%) women. The median age was 70 years (IQR = 17). The reference group consisted of 441 subjects: 213 (48.3%) men and 228 (51.7%) women; the median age within the group was 69 years (IQR = 18) (Table S1).
The analysis of VEGFA (rs1570360, rs699947, rs3025033, and rs2146323) showed that the rs3025033 GG genotype was less frequent in the periodontitis group than in the reference group (1.6% vs. 5.7%, p = 0.008) ().
Table 1. Genotype and allele distribution of VEGFA (rs1570360, rs699947, rs3025033, and rs2146323) in periodontitis and reference groups.
Binary logistic regression analysis was performed to evaluate the presence of rs1570360, rs699947, rs3025033, and rs2146323 in periodontitis. The analysis revealed that the VEGFA rs3025033 GG genotype was associated with a 4-fold decreased odds of periodontitis under the codominant model (OR = 0.25; CI: 0.09–0.73, p = 0.011) (). We have also prepared a binary logistic regression analysis between periodontitis and the reference group when age and gender were selected as covariates. However, these results did not show statistically significant results after the Bonferroni correction (Supplementary material Table S2).
Table 2. Binomial logistic regression analysis of periodontitis and reference groups.
Genotype and allele distribution of VEGFA SNPs (rs1570360, rs699947, rs3025033, and rs2146323) was performed separately between males and females. The analysis did not reveal any statistically significant results (Supplementary material Table S3 and Table S4). Binary logistic regression analysis was performed to evaluate the association of VEGFA (rs1570360, rs699947, rs3025033, and rs2146323) on periodontitis development in males (Table S5) and in females (Table S6). However, the analysis did not reveal any statistically significant results after the Bonferroni correction (p > 0.05/4).
Also, study groups were divided in two groups by median age (>70 years old, and < =70 years old). However, after Bonferroni correction, the analysis revealed no statistically significant results between periodontitis and reference group subjects compared in age groups. The results are presented in the supplementary material (Table S7 – Table S10).
VEGFA serum levels in periodontitis patients and reference group subjects
We found that VEGFA serum levels were not statistically significantly different between periodontitis patients and reference group subjects (554.29 (522.38) ng/ml vs. 581.32 (348.16) ng/ml, p = 0.786) ().
Figure 2. VEGFA serum levels in periodontitis and reference group subjects. Mann-Whitney U test was used to Assess serum. (A) VEGFA serum levels comparison between periodontitis and reference group subjects. (B) VEGFA serum levels comparison between females and males in reference group. (C) VEGFA serum levels comparison between females and males in periodontitis group.

Analysis showed elevated VEGFA serum levels in reference group females compared to males (median (IQR): 598.15 (425.55) ng/mL vs. 402.65 (481.61) ng/mL, p = 0.047) (). However, no statistically significant results were found between females and males in periodontitis group (median (IQR): 597.67 (832.85) ng/mL vs. 313.38 (314.86) ng/mL, p = 0.076) ().
Serum VEGFA levels comparison between study groups and genotypes was performed; Analysis revealed lower VEGFA serum levels in the periodontitis subjects with rs1570360 GG genotype compared to reference group subjects (median (IQR): 315.06 (297.64) ng/mL vs. 508.64 (414.46) ng/mL, p = 0.033) (Table S11).
We performed haplotype analysis of VEGFA (rs1570360, rs699947, rs3025033, and rs2146323) polymorphisms. Pairwise linkage disequilibrium (LD) between studied polymorphisms was observed. The deviation between the predicted haplotype frequency and the observed frequency (D’) was calculated, and the square of the correlation coefficient (r2) was estimated. Data are presented in Table S12.
We analyzed haplotype frequencies, and the statistical analysis of periodontitis showed that individuals carrying rs1570360, rs699947, rs3025033, and rs2146323 haplotype A-A-G-A had decreased risks of periodontitis, while rare haplotype of VEGFA (rs1570360, rs699947, rs3025033, and rs2146323) was associated with the increased odds of periodontitis (OR = 0.42; 95% CI: 0.20 − 0.85; p < 0.017; OR = 6.01; 95% CI: 2.19 − 16.45; p < 0.0001, respectively) ().
Table 3. Haplotype association with periodontitis.
Discussion
This study analyzed four SNP’s in the VEGFA gene and their association with periodontitis. Our study revealed that the rs3025033 GG genotype was associated with a lower probability of developing periodontitis. Importantly, analysis of haplotype frequencies revealed, that individuals carrying rs1570360, rs699947, rs3025033, and rs2146323 haplotype A-A-G-A had a lower risk of periodontitis. However, a rare haplotype of VEGFA (rs1570360, rs699947, rs3025033, and rs2146323) was found to be associated with increased odds of periodontitis development.
It is known, that VEGF is the most potent angiogenic and vasculogenic factor involved in tertiary dentine formation. VEGF, an endothelial-specific secreted protein, plays an important role in angiogenesis [Citation26]. VEGF has been reported to be expressed in human pulp and to play an autocrine and paracrine role in local blood vessels and immune cells [Citation27]. Sakai et al. in a previous study, pulpal stem cells were found to secrete VEGF during activation [Citation28], and VEGF was found to induce the proliferation and differentiation of human pulp cells into odontoblasts and may be a useful growth factor in the repair of damaged pulp and dentine [Citation29]. Although no scientific literature data were found for the above-mentioned SNPs in the VEGFA gene and their association with periodontitis, scientists Balci et al. found that VEGF expression may be present during periodontal inflammation [Citation30]. However, there were no differences in VEGF expression in gingival biopsy specimens from 15 systemically and orally healthy individuals, 15 systemically healthy periodontitis patients, 15 systemically and orally healthy smokers, and 15 systemically healthy smoker periodontitis patients who participated in the present study [Citation31]. It is important to note that inconsistencies regarding VEGFA expression have been found in various studies [Citation30,Citation31]. A study by Ramya et al. that examined patients with type 2 diabetes with and without periodontitis concluded that periodontal disease may have an effect on VEGFA levels [Citation32].
Although sufficient evidence for VEGFA expression in periodontal patients was not found in the scientific literature, only studies analyzing nearby anatomical regions were found. In a research done by Zhang et al., it was found, that VEGFA had a positive effect in enhancing pulp cell proliferation and neovascularization, along with the formation of reparative dentine [Citation33]. Other scientists group have noted, that VEGFA differential expression was superior in platelet rich fibrine (PRF) in comparison to platelet-rich fibrin matrix (PRFM) and dental pulp, which could be beneficial in regards to PRF use for pulp vascularity restoration and pulpal healing [Citation34]. Also, in a research done by Sosa et al. it was found, that the expression of VEGFA and its receptors is significantly higher in mature apex teeth, in comparison to the immature apex teeth [Citation35]. When analyzing VEGFA expression immunohistochemical data, it was noted, that the expression was strongly positive in the inflammatory infiltrate of pulpitis cases [Citation36].
Nevertheless, when analysing VEGFA role in pharyngeal tissue of patients, affected by obstructive sleep apnoea hypopnoea syndrome, scientists Zeng et al. noted the great importance of VEGF expression and relationship with the degree of hypoxia in patients affected by this syndrome [Citation37]. Safak et al. have also found that VEGF expression of the tonsiliar surface epithelium increases in patients [Citation38].
In addition, favourable results were found when neighbouring regions where diseases were associated with VEGFA polymorphisms were analyzed. Dimitrakopoulos et al. have reported, that VEGFA rs13207351 was the most clinically promising polymorphism in relation to the Head and Neck Cancer (HNC) prognosis [Citation39]. Chen et al. have found, that VEGFA expression could be an indication of a low survival rate in oesophageal carcinoma [Citation40]. In contrast, Formento et al. investigated that VEGFA 936 C > T may not be a prognostic factor in head and neck cancer patients [Citation41]. Although Xu et al. performed a meta-analysis in which they analyzed VEGFA +936 C > T polymorphisms, the results showed that there were no differences between T and C despite the existing association [Citation42]. However, a study on the association with HNC concluded that the aforementioned polymorphisms were not associated with the disease in both Asians and Caucasians [Citation43].
Researchers have found that VEGFA polymorphisms have a significant association with the risk of oesophageal cancer (EC) [Citation44, Citation45]. Eng et al. conducted a study in which they found no association between VEGFA polymorphisms and overall survival and progression-free survival of patients with oesophageal cancer [Citation46]. However, Yang, et al. represented different results, with VEGFA rs2010963 polymorphism showing an association with a better overall survival rate of patients with advanced oesophageal squamous cell carcinoma [Citation47].
When VEGFA polymorphisms were analyzed, they were also found to be associated with the risk of nasopharyngeal carcinoma (NPC). Scientists Wang et al. investigated that patients with the VEGFA − 2578 A allele had a significant association with an increased risk of NPC [Citation48]. Moreover, VEGFA − 1154 GG was found to be a poor prognostic marker for survival in patients suffering from oral squamous cell carcinoma [Citation49]. Moreover, Psoma et al. have concluded, that VEGFA polymorphisms might also be associated with the clinical prognosis of NPC patients [Citation50].
The following studies show that VEGFA polymorphisms are both a prognostic marker and an inflammatory component for several diseases, including periodontitis. The results suggest mixed conclusions, as further studies are required for additional results, with larger populations and VEGFA polymorphisms assessment in the periodontitis field.
Several limitations of the present study need to be considered. We did not include periodontitis stages, and we did not analyze periodontitis stages in relation to SNP. Furthermore, the analysis of important aetiological factors in periodontitis development, i.e. smoking and alcohol consumption, was not carried out in the present study. However, this is foreseen as a targeted task in future investigations.
Conclusions
The rs3025033 GG genotype and the rs1570360, rs699947, rs3025033, and rs2146323 A-A-G-A haplotypes may play a protective role in the development of periodontitis, but a rare haplotype of the same VEGFA polymorphisms may be associated with the risk of developing periodontitis.
Informed consent statement
Informed consent was obtained from all subjects involved in the study.
Institutional review board statement
The Ethics Committee approved the study for Biomedical Research at the Lithuanian University of Health Sciences (No. BE −2-20). All subjects gave written informed consent in accordance with the Declaration of Helsinki.
Supplemental Material
Download MS Word (71.2 KB)Disclosure statement
The authors report there are no competing interests to declare.
This research received no external funding.
References
- Kassebaum NJ, Bernabé E, Dahiya M, et al. Global burden of severe periodontitis in 1990-2010: a systematic review and meta-regression. J Dent Res. 2014;93(11):1045–1053. doi: 10.1177/0022034514552491.
- Kassebaum NJ, Smith AGC, Bernabé E, et al. Global, regional, and national prevalence, incidence, and disability-adjusted life years for oral conditions for 195 countries, 1990–2015: a systematic analysis for the global burden of diseases, injuries, and risk factors. J Dent Res. 2017;96(4):380–387. doi: 10.1177/0022034517693566.
- Baima G, Romandini M, Citterio F, et al. Periodontitis and accelerated biological aging: a geroscience approach. J Dent Res. 2022;101(2):125–132. doi: 10.1177/00220345211037977.
- Ioannidou E. The sex and gender intersection in chronic periodontitis. Front Public Health. 2017;5:189. doi: 10.3389/fpubh.2017.00189.
- Luis Muñoz-Carrillo J, Elizabeth Hernández-Reyes V, Eduardo García-Huerta O, et al. Pathogenesis of periodontal disease. In: ; Mohammed Ahmed Yussif, N., editor. Periodontal Disease - Diagnostic and adjunctive non-surgical considerations.; IntechOpen; 2020 ISBN 978-1-78984-460-3. doi: 10.5772/intechopen.86548.
- Hajishengallis G, Lamont RJ. Beyond the red complex and into more complexity: the polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol Oral Microbiol. 2012;27(6):409–419. doi: 10.1111/j.2041-1014.2012.00663.x.
- Hajishengallis G. The inflammophilic character of the periodontitis-associated microbiota. Mol Oral Microbiol. 2014;29(6):248–257. doi: 10.1111/omi.12065.
- Celik D, Kantarci A. Vascular changes and hypoxia in periodontal disease as a link to systemic complications. Pathogens. 2021;10(10):1280. doi: 10.3390/pathogens10101280.
- da Silva MK, de Carvalho ACG, Alves EHP, et al. Genetic factors and the risk of periodontitis development: findings from a systematic review composed of 13 studies of meta-analysis with 71,531 participants. Int J Dent. 2017;2017:1914073–1914079. doi: 10.1155/2017/1914073.
- Moens S, Goveia J, Stapor PC, et al. The multifaceted activity of VEGF in angiogenesis – implications for therapy responses. Cytokine Growth Factor Rev. 2014;25(4):473–482. doi: 10.1016/j.cytogfr.2014.07.009.
- Takahashi H, Shibuya M. The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Clin Sci. 2005;109(3):227–241. doi: 10.1042/CS20040370.
- Tischer E, Mitchell R, Hartman T, et al. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem. 1991;266(18):11947–11954.
- Andrikopoulos P, Fraser SP, Patterson L, et al. Angiogenic functions of voltage-gated Na + channels in human endothelial cells. J Biol Chem. 2011;286(19):16846–16860. doi: 10.1074/jbc.M110.187559.
- Lucarini G, Tirabassi G, Zizzi A, et al. Uncoupling of vascular endothelial growth factor (VEGF) and inducible nitric oxide synthase (INOS) in gingival tissue of type 2 diabetic patients. Inflammation. 2016;39(2):632–642. doi: 10.1007/s10753-015-0288-9.
- Johnson RB, Serio FG, Dai X. Vascular endothelial growth factors and progression of periodontal diseases. J Periodontol. 1999;70(8):848–852. doi: 10.1902/jop.1999.70.8.848.
- Tayman MA, Kurgan Ş, Önder C, et al. Disintegrin‐like and metalloproteinase with thrombospondin‐1 (ADAMTS‐1) levels in gingival crevicular fluid correlate with vascular endothelial growth factor‐A, hypoxia‐inducible factor‐1α, and clinical parameters in patients with advanced periodontitis. J Periodontol. 2019;90(10):1182–1189. doi: 10.1002/JPER.18-0195.
- Padma R, Sreedhara A, Indeevar P, et al. Vascular endothelial growth factor levels in gingival crevicular fluid before and after periodontal therapy. J Clin Diagn Res. 2014;8:75–79.
- Tian Y, Li J, Hao L, et al. Association of cytokines, high sensitive C-reactive protein, VEGF and Beta-Defensin-1 gene polymorphisms and their protein expressions with chronic periodontitis in the Chinese population. Int J Biol Markers. 2013;28(1):100–107. doi: 10.5301/jbm.5000010.
- Lin ZJ, Luo RH, Xie LL, et al. Effects of basic periodontal treatment on endothelin, vascular endothelial growth factor-A and tumor necrosis factor-α levels in gingival crevicular fluid and serum. Shanghai Kou Qiang Yi Xue. 2019;28(5):504–508.
- Afacan B, Keleş Yücel ZP, Paşali Ç, et al. Effect of non‐surgical periodontal treatment on gingival crevicular fluid hypoxia inducible factor‐1 alpha, vascular endothelial growth factor and tumor necrosis factor‐alpha levels in generalized aggressive periodontitis patients. J Periodontol. 2020;91(11):1495–1502. doi: 10.1002/JPER.19-0521.
- Kwon T, Lamster IB, Levin L. Current concepts in the management of periodontitis. Int Dent J. 2021;71(6):462–476. doi: 10.1111/idj.12630.
- Taskan MM, Karatas O, Balci Yuce H, et al. Hypoxia and collagen crosslinking in the healthy and affected sites of periodontitis patients. Acta Odontol Scand. 2019;77(8):600–607. doi: 10.1080/00016357.2019.1624819.
- ClinVar aggregates information about genomic variation and its relationship to human health. https://www.ncbi.nlm.nih.gov/clinvar/.
- Papapanou PN, Sanz M, Buduneli N, et al. Periodontitis: consensus report of workgroup 2 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J Periodontol. 2018;89(Suppl 1):S173–S182.
- Web tool for SNP analysis. SNPStats. Available at: https://www.snpstats.net/start.htm. (Accessed: 2 April 2023).
- Hood JD, Meininger CJ, Ziche M, et al. VEGF upregulates EcNOS message, protein, and NO production in human endothelial cells. Am J Physiol. 1998;274(3):H1054–H1058. doi: 10.1152/ajpheart.1998.274.3.H1054.
- Virtej A, Løes S, Iden O, et al. Vascular endothelial growth factors signalling in normal human dental pulp: a study of gene and protein expression. Eur J Oral Sci. 2013;121(2):92–100. doi: 10.1111/eos.12019.
- Sakai VT, Zhang Z, Dong Z, et al. SHED differentiate into functional odontoblasts and endothelium. J Dent Res. 2010;89(8):791–796. doi: 10.1177/0022034510368647.
- Matsushita K, Motani R, Sakuta T, et al. The role of vascular endothelial growth factor in human dental pulp cells: induction of chemotaxis, proliferation, and differentiation and activation of the AP-1-Dependent signaling pathway. J Dent Res. 2000;79(8):1596–1603. doi: 10.1177/00220345000790081201.
- Balci Yuce H, Karatas Ö, Tulu F, et al. Effect of diabetes on collagen metabolism and hypoxia in human gingival tissue: a stereological, histopathological, and immunohistochemical study. Biotech Histochem. 2019;94(1):65–73. doi: 10.1080/10520295.2018.1508745.
- Karatas O, Balci Yuce H, Tulu F, et al. Evaluation of apoptosis and hypoxia-related factors in gingival tissues of smoker and Non-Smoker periodontitis patients. J Periodontal Res. 2020;55(3):392–399. doi: 10.1111/jre.12723.
- Ramya M, Kumar S. Expression of VEGF in periodontal tissues of type II diabetes mellitus patients with chronic periodontitis -an immunohistochemical study. J Clin Diagn Res. 2014;8: ZC01–03.
- Zhang J, Liu X, Yu W, et al. Effects of human vascular endothelial growth factor on reparative dentin formation. Mol Med Rep. 2016;13(1):705–712. doi: 10.3892/mmr.2015.4608.
- Nagaraja S, Mathew S, Abraham A, et al. Evaluation of vascular endothelial growth factor - A release from platelet-rich fibrin, platelet-rich fibrin matrix, and dental pulp at different time intervals. J Conserv Dent. 2020;23(4):359–363. doi: 10.4103/JCD.JCD_465_19.
- Gomez-Sosa JF, Caviedes-Bucheli J, Barrera L. Gene expression of vascular endothelial growth factor a and its receptors in dental pulp of immature and mature teeth. Eur Endod J. 2021;6(3):259–263. doi: 10.14744/eej.2021.86580.
- Artese L, Rubini C, Ferrero G, et al. Vascular endothelial growth factor (VEGF) expression in healthy and inflamed human dental pulps. J Endod. 2002;28(1):20–23. doi: 10.1097/00004770-200201000-00005.
- Zeng HH, Dong W, Xie YP, et al. Cyclooxygenase-2 overexpression and vascular endothelial growth factor expression in pharyngeal tissue of patients with OSAHS correlates with angiogenesis. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2011;27(2):210–213.
- Safak AS, Bulut F, Cumbul A. Histopathological role of vitamin D deficiency in recurrent/chronic tonsillitis pathogenesis: vascular epithelial growth factor‐mediated angiogenesis in tonsil. Clin Exp Dent Res. 2022;8(3):699–706. doi: 10.1002/cre2.539.
- Dimitrakopoulos FI, Koliou GA, Kotoula V, et al. Genetic variation in the vascular endothelial growth factor (VEGFA) gene at Rs13207351 is associated with overall survival of patients with head and neck cancer. Cancers. 2021;13(5):1163. doi: 10.3390/cancers13051163.
- Chen M, Cai E, Huang J, et al. Prognostic value of vascular endothelial growth factor expression in patients with esophageal cancer: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2012;21(7):1126–1134. doi: 10.1158/1055-9965.EPI-12-0020.
- Formento JL, Etienne-Grimaldi MC, Francoual M, et al. Influence of the VEGF-A 936C > T germinal polymorphism on tumoral VEGF expression in head and neck cancer. Pharmacogenomics. 2009;10(8):1277–1283. doi: 10.2217/pgs.09.54.
- Xu B, Li JM, Tong N, et al. VEGFA +936C > T polymorphism and cancer risk: a meta-analysis. Cancer Genet Cytogenet. 2010;198(1):7–14. doi: 10.1016/j.cancergencyto.2009.11.007.
- Leng WD, He MN, Chen QL, et al. Vascular endothelial growth factor (VEGF) gene polymorphisms and risk of head and neck cancer: a meta-analysis involving 2,444 individuals. Mol Biol Rep. 2013;40(10):5987–5992. doi: 10.1007/s11033-013-2708-y.
- Guleria K, Kaur S, Mahajan D, et al. Impact of VEGFA promoter polymorphisms on esophageal cancer risk in North-West Indians: a case-control study. Genes Genomics. 2022;44(8):923–936. doi: 10.1007/s13258-022-01269-2.
- Qasim I, Bhat IA, Masoodi KZ, et al. Role of +405C > G and +936C > T polymorphisms of the vascular endothelial growth factor gene and risk of esophageal cancer in the Kashmiri population. Asian Pac J Cancer Prev. 2015;16(1):97–101. doi: 10.7314/apjcp.2015.16.1.97.
- Eng L, Azad AK, Qiu X, et al. Discovery and validation of vascular endothelial growth factor (VEGF) pathway polymorphisms in esophageal adenocarcinoma outcome. Carcinogenesis. 2015;36(9):956–962. doi: 10.1093/carcin/bgv073.
- Yang PW, Hsieh MS, Huang YC, et al. Genetic variants of EGF and VEGF predict prognosis of patients with advanced esophageal squamous cell carcinoma. PLoS One. 2014;9(6):e100326. doi: 10.1371/journal.pone.0100326.
- Wang T, Hu K, Ren J, et al. Polymorphism of VEGF-2578C/a associated with the risk and aggressiveness of nasopharyngeal carcinoma in a chinese population. Mol Biol Rep. 2010;37(1):59–65. doi: 10.1007/s11033-009-9526-2.
- Supic G, Jovic N, Zeljic K, et al. Association of VEGF-A genetic polymorphisms with cancer risk and survival in advanced-stage oral squamous cell carcinoma patients. Oral Oncol. 2012;48(11):1171–1177. doi: 10.1016/j.oraloncology.2012.05.023.
- Psoma E, Koliou GA, Dimitrakopoulos FI, et al. Genetic variations of VEGFA gene are associated with infiltration of adjacent tissues and the clinical outcome of patients with nasopharyngeal carcinoma. Anticancer Res. 2020;40(2):677–688. doi: 10.21873/anticanres.13997.