ABSTRACT
This study establishes the peculiarities of the major parameters of reproductive biology (features of the structures and processes in the male and female generative sphere, pollen and seed viability) of three species of genus Colchicum from the Bulgarian flora – Colchicum autumnale, Colchicum diampolis and Colchicum bivonae. The anthers are tetrasporangiate. Their wall develops according to monocotyledonous type and consists of epidermis, fibrous endothecium, two middle layers and glandular tapetum. The sporogenous tissue in the anther locule is one-layered. The microspore tetrads formed after regular meiosis in microspore mother cells and successive microsporogenesis are predominantly tetrahedral. The mature pollen grains are two-celled. The ovule is anatropous, medionucellate and bitegmic. In it a unicellular archesporium forms without cutting off parietal cells. The embryo sac develops after Polygonum (monosporic)-type. The embryogenesis follows the Asterad-type. The formation of an additional embryo derived from one of the synergids was found in C. diampolis. The endospermogenesis passes a free nuclear stage before its transformation in cellular one. The established high pollen and seed viability provides the investigated Colchicum species with high reproductive capacity.
1. Introduction
Reproductive biology has important significance for the state of populations of rare and endemic plants. The distribution of these kinds of species requires that common conservation strategies be sought, and investigations should be focused on application of strategies based on multiple aspects of population biology of these plants. Knowledge of several aspects of population-reproductive biology is necessary to predict risks to population viability of rare species (Evansa et al. Citation2003). The study of the reproductive biology of endemic and rare species has an important role in determining its dissipation and distribution in environmental conditions, by establishment in type of reproduction, characteristics of reproductive structures and processes involved in reproduction because the amount and quality of “breeding units” define the reproductive capacity and state of their populations.
In this work are presented the results of the study of the peculiarities of the reproductive biology of three species of genus Colchicum distributed in Bulgaria: C. autumnale, C. diampolios and C. bivonae. They belong to the family Colchicaceae (formerly Liliaceae).
Colchicum autumnale L. is known as an important medicinal plant worldwide. Modern medicine uses Colchicum as a source of therapeutically active alkaloids – colchicinoids. One of the most abundant alkaloids, colchicine, is known to have cancerostatic, antirheumatic, anti-inflammatory, antimitotic, cathartic and emetic effects (Boyé and Brossi Citation1992; Katzung Citation2004). These alkaloids are contained predominantly in the roots and seeds – the parts of the plant used for their medicinal properties. Despite its common spread, C. autumnale can be found in Red Data Books of several countries at the distribution limits: Great Britain, Ireland, the Netherlands, Luxembourg, Lithuania, Estonia, Belarus, Ukraine, Albania, and the northern federal states of Germany. A decline in the number of sites has been noted in Poland and Ukraine mainly as a result of intensification of agriculture, draining, ploughing and re-seeding grassland (Jung et al. Citation2011).
In Bulgaria C. autumnale is not protected but it is recommended during its collection to leave a part of the seminal boxes for reproduction via seeds, to allow collection of seeds from same locality for at least two years.
Colchicum bivonae Guss. is an endangered species protected under the Biodiversity Act (Citation2002). Parts of the habitats of the species are included in the protected areas of European ecological network NATURA 2000 in Bulgaria. Negative factors that impact the state of populations of the species are: destruction of specific habitats; limited distribution and habitats anthropogenically impacted; low population density and low regenerative ability (Bancheva Citation2010a).
Colchicum diampolis Delip. et Ceschm. is a Bulgarian endemic critically endangered species protected under the Biodiversity Act (Citation2002). It is included in the list of threatened plants of the IUCN in 1997 with category “rare”. Some of the populations of the species are within the protected area “Ormana” near the town Yambol. Negative factors that impact the state of populations of the species are: destruction of the habitats of the species; attachment to a specific habitat and low migration capabilities; exclusively rare and highly fragmented habitats (Bancheva Citation2010b).
This is the first time this kind of study has been carried out on a Bulgarian population of the cited species, as well as on species of genus Colchicum at all. It was focused on the following main points concerning the reproductive biology of the studied species: (1) To establish the mode of reproduction of their native populations in Bulgaria; (2) To characterize the peculiarities of their reproductive structures and the processes running in them; (3) To estimate their pollen and seed (embryo) viability.
2. Materials and methods
2.1. Plant material
One native population of Colchicum autumnale and Colchicum bivonae and two of Colchicum diampolis were studied ().
Table 1. Origin of plant material.
Table 2. Pollen viability in studied species, analysed by acetocarmine staining.
From the cited populations were collected plant specimens deposited in the herbaria in Sofia (SOM) and Plovdiv (SOA).
2.2. Study of the reproductive capacity
For the evaluation of the reproductive potential of the studied species, three major parameters of their reproductive biology were studied: the features of the reproductive sphere, pollen viability, and seed viability.
For the purposes of the embryological study flower buds and flowers at different developmental stages were collected and fixed in a mixture of FAA (formalin:glacial acetic acid: 70% ethanol in correlation 5:5:90 parts) and treated according to classical paraffin method (Sundara Citation2000), namely: embedded in paraffin, and cut up into 8–12 μm sections with a Leica rotary microtome. The sections obtained were laid on slides, stained with Heidenhain’s haematoxylin and included in Entellan.
To estimate the pollen viability the acetocarmine stain technique was used (Heslop-Harrison Citation1992). Anthers from the open flowers were collected and suspended in a solution containing acetocarmine – 1% acetocarmine was used for staining (Singh Citation2003). The pollen viability was estimated on temporary slides taking into account the colouring of mature pollen from the acetocarmine: stained red – viable, fertile; and unstained – nonviable, sterile. The viability was expressed in percentages on the base of counting using a light microscope the mature pollen grains in 30 anthers on a visual field, at a magnification of ×100. The total number of pollen grains examined was 735 ().
To estimate the seed (embryo isolated from the seed) viability, a tetrazolium test (Peters Citation2000) was applied. It is based on the ability of the tetrazolium solution to change from colourless to red when it contacts with the hydrogen emitted during the respiratory process of the embryos. Embryos showing active respiration turn red and are considered viable. For the test purposes, approximately 200 mature seeds of each population of studied species were preliminarily incubated in water for 24 h at 30–35°C. Then the seeds were cut deeply in its chalazal part and incubated in a diluted (1%) solution of 2,3,5-triphenyltetrazolium chloride for 24 h. The quality of mature embryos (their viability potential) was determined by observations of the staining patterns of embryos and the intensity of staining: viable embryos display entire embryo staining or staining of its basal part – the root (normal staining); nonviable embryos display abnormal or no staining. The manner of staining reveals the live and dead areas of the embryos and allows it to be determined whether the seeds have the capacity to produce normal seedlings (Copeland and McDonald Citation2001).
The observations carried out were made using an Olympus CX21 light microscope (Olypus Corporation, Tokyo, Japan) and micrographs – with “Infinity lite” digital camera (1.4 Mpx) (Lumenera Corporation, Ottawa, Ontario, Canada).
3. Results
3.1. Embryological features
3.1.1. Anther and development of the male gametophyte
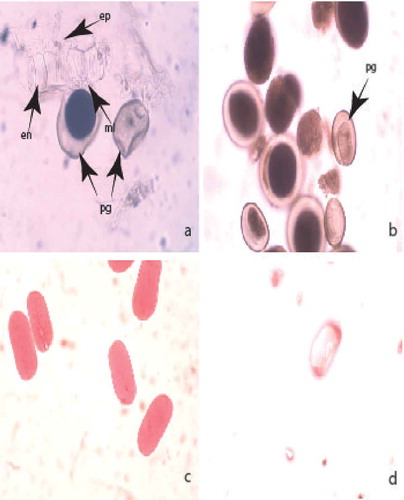
The anthers are tetrasporangiate. The wall formation runs according to monocotyledonous type. It consists of five layers: an epidermis, an endothecium, two middle layers and a tapetum. The epidermis comprises one row of almost rectangular uninucleate cells that during the anther’s ontogenesis vastly enlarge tangentially. One of the middle layers is ephemeral and completely degenerates up to the end of meiosis in microspore mother cells (MMCs); the other remains viable up to the mature pollen stage. At the beginning of the anther’s ontogenesis, the endothecium cells are similar in size and shape to the epidermal ones. Subsequently, they radially elongate and develop fibrous thickenings after the stage of one-nucleated pollen. The tapetum is one-layered, glandular and remains such during the whole anther ontogenesis. At the stage of mature pollen, the anther wall consists of epidermis, endothecium and one of the middle layers partially conserved (()).
The sporogenous tissue is one-rowed. Initially, its cells are polygonal and fit close to each other. Later on they elongate, round up, separate from each other and function as MMCs. The meiosis in MMCs passes with insignificant deviations. After successive microsporogenesis, predominantly tetrahedral microspore tetrads form. At the time of shedding the pollen grains are two-celled and morphologically uniform (()).
Figure 1. Development of male gametophyte and estimation of pollen viability by acetocarmine testing: (a) mature pollen and anther wall; (b) two-celled mature pollen grains; (c) viable pollen grains; (d) nonviable pollen grain. Abbreviations: pg, pollen grain; ep, epiderma; en, endotecium; ml middle layer. Magnification: 400×.
3.1.2. Ovule and development of the female gametophyte
The pistil is syncarpous and superior. In the three-locular ovary, 20–25 ovules with axile placentation form in each locule (()). The ovule is anatropous, with 3–4 layered nucelli and two integuments (, ). The micropyle is formed only by the inner integument (, ) composed from two to three cell layers. The archesporium is one-celled and the archesporium cell differentiates in the megaspore mother cell without cutting off parietal cells. After meiosis running in it, a linear megaspore tetrad forms. The embryo sac (ES) development runs according to the Polygonum (monosporic)-type from the chalazal megaspore of tetrad. This cell enlarges and becomes an ES mother cell. The other three megaspores degenerate progressively, during the ES development. The mature ES consists of three-celled egg apparatus (a pear-shaped egg cell and two synergids), two polar nuclei and three-celled antipodal apparatus. The synergids are not hooked and do not form a filiform apparatus. They degenerate after fertilization. The polar nuclei and the central cell formed as a result of their fusion are located close to the egg cell (, )). The antipodals, usually in T-shape arrangement, are located on a podium formed from the nucellar cells of the chalazal part of ES (). They do not proliferate during the female gametophyte development and persist after fertilization and young embryo formation ()).
Figure 2. Ovule and development of female gametophyte: (a) three-locular ovary; (b) mature embryo sac with nucellar cap and integumental tapetum and egg apparatus; (c) egg apparatus and two polar nuclei in the mature embryo sac; (d) egg apparatus and central cell in the mature embryo sac. Abbreviations: hyp, hypostase; it, integumental tapetum; nc, nucellar cap; pn, pollar nucleus; cc, central cell; egc, egg cell; sin, synergid. Magnification: 100× for (a), 400× for (b–d).
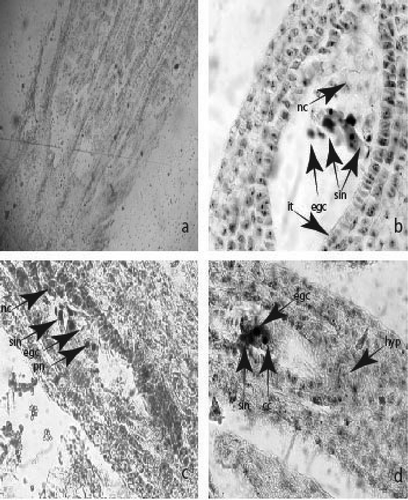
Figure 3. Ovule and development of female gametophyte: (a) two celled proembryo and endospermal nuclei; (b) young Asterad-type embryo in the embryo sac; (c) globular embryo; (d) mature embryo sac with podium, hypostase and egg cell, antipodals and additional synergid embryo. Abbreviations: egc, egg cell; ant, antipodal; em, embryo; endn, еndospermal nucleus; hyp, hypostase. Magnification 400×.
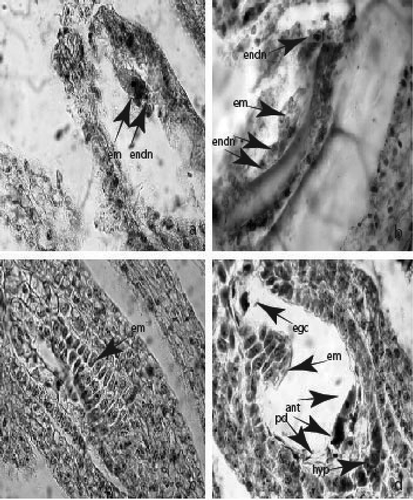
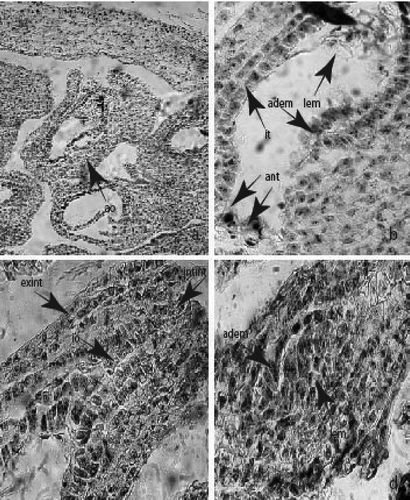
Figure 4. Ovule and development of female gametophyte: (a) anatropous ovule in the ovary locule; (b) mature embryo sac with integumental tapetum and legitimate, additional embryo and antipodals (c) integumental obturator; (d) two embryos – legitimate and additional at globular stage. Abbreviations: ao, anatropous ovule; io, integumental obturator; it, integumental tapetum; ant, antipodal; lem, legitimate embryo; adem, additional embryo; intint, internal integument; exint, external integument. Magnification: 100x for fig. (a), 400x for figs (b)-(d).
In the nucellus of the ovules of the three studied species, other specific structures beside the podium were observed as well: a nucellar cap – the result of peryclinal divisions of the cells of the nucellar epidermis ()) and a hypostase – formed of a group of cells in the form of a disk in the chalazal part of ES ()).
During the study it was observed that at the stage of two nucleate ES the cells of the innermost layer of the inner integument of the ovule enlarge radially and become distinguishable from the other integumental layers. These cells form a structure surrounding the ES, known as the integumental tapetum. It persists during the ES development and degenerates after fertilization ()).
Another specific structure established in the ovule of the studied Colchicum species was the integumental obturator – composed of elongated epidermal cells of the outer integument that intrudes deeply into the micropyle ())
The embryo and the endosperm develop after porogamous double fertilization of the egg cell and central cell from the sperms of pollen grains penetrating in ES cavity through the pollen tube. The first division of the zygote (resulting of the fusion of the one sperm of pollen grain with the egg cell) is transversal and runs after the beginning of endospermogenesis ()). The direction of the cell wall setting after the next divisions leading to formation of the young embryo indicates that the embryogenesis occurs according to Asterad-type embryo formation ()). The globular embryo has a not very long massive suspensor consisting of two rows of rectangular cells ()). The mature embryo is straight with one cotyledon.
The endosperm (forming after fusion of the second sperm of pollen grain with the central cell) is cellular, but passes a nuclear stage which coincides with the phases of young embryo formation ()). It persists in the mature seed.
In C. diampolis the formation of an additional embryo from the one synergid was observed ()). The additional globular embryo has a long one-layered suspensor ()).
3.2. Pollen and seed viability
On the base of colouring by the acetocarmine solution after acetocarmine testing, the mature pollen grains of the studied species were estimated as viable (stained in red – )) and nonviable (colourless – )). The calculated percentages of pollen viability were high (over 80% – ).
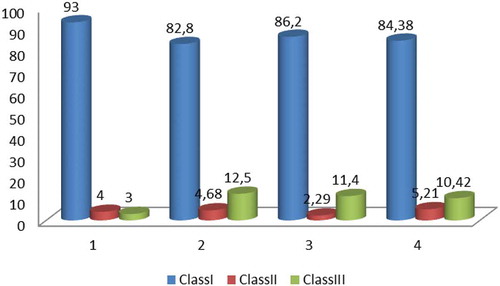
The seeds (embryos) were differentiated in three classes on the basis of colour patterns resulting after application of tetrazolium test (Figures 5 and 6): Class I – embryos stained 100% (whole embryo stained in dark red – , )); Class II – embryos stained 80% (light red coloured embryos – )); Class III – colourless embryos ()). According to the criteria for interpretation of the results of tetrazolium test given by Moore (Citation1985), the viable embryos are represented by the embryos of Classes I and II (). The resulting viability was as follows: 97% in the population of C. autumnale; in C. diampolis – 88.6% (population from Karnobat) and 87.5% (population from Yambol); 89.58% – in the population of C. bivonae. In summary, the established seed viability in the studied Colchicum species can be defined as high.
Figure 5. Seed viability according tetrazolium test: (a) dark red coloured viable embryo; (b) dark red coloured viable embryo with part of endosperm; (c) light red coloured viable embryo; (d) nonviable colourless embryo. Abbreviations: cot, cotyledon; end, endosperm; rt, root tip.
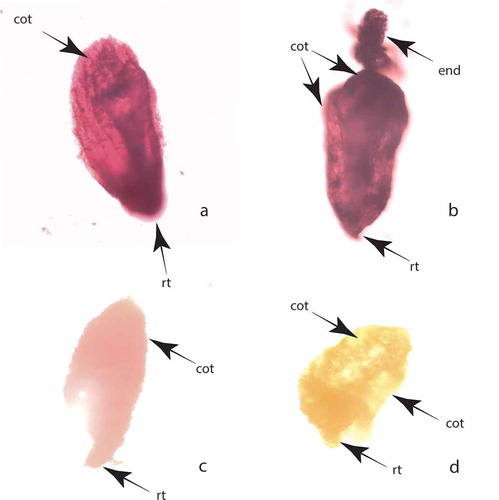
Figure 6. Frequency of embryos in classes depending on the stainability in the three studied species: Class I – dark red coloured embryos; Class II – light red coloured embryos; Class III – stainless embryos; 1. C. autumnale; 2. C. diampolis – population from Karnobat; 3. C. diampolis – population from Yambol; 4. C. bivonae.
4. Discussion
The structures and processes in generative sphere of the flower of three studied species of Colchicum leading to formation of seeds follow the characteristics established in the family Liliaceae/Colchicaceae (Davis Citation1966; Poddubnaya-Arnoldi Citation1982; Watson and Dallwitz Citation1992).
During the study it was established that the gynoecium is syncarpous, superior – typical for the family Colchicaceae (Nordenstam Citation1998, Watson & Dawliz Citation1992). The observed trilocular ovary is consistent with the 3–4 loculate ovary reported for this family by Nordenstam (Citation1998) and Watson and Dawliz (Citation1992). For this family, anatropous, campylotropous and hemianatropous ovules were described (Davis Citation1966; Poddubnaya-Arnoldi Citation1982; Watson and Dawliz 1992). Our observations show that the ovule in the studied species is anatropous. Regarding the structure (development and structure of nucellus), the observed ovules in the family were defined as tenuinucellates and crassinucellates (Davis Citation1966; Poddubnaya-Arnoldi Citation1982; Watson and Dawliz 1992). The presence of two integuments and a well-developed nucellus characterize the ovule in the studied species as crassinucellate, like the majority of monocotyledons (Rudall Citation1997), which are generally considered to be bitegmic and crassinucellate on the basis of plesiomorphic conditions in the ovule structure (Palser Citation1975). According to the more detailed classification of ovule types given by Shamrov (Citation1999) which introduces a new medionucellate type, it can be referred to as the medionucellate type, “syndermal” variation, “multilayered” subvariation. This type the author refers to the monocots, including Liliaceae. In the aforementioned type the nucellus is presented from its basal and lateral areas that are multilayered and have a massive nucellar cap. According to Shamrov in this ovule type the integumental tapetum does not differentiate. However, we have established the differentiation of such structure in the studied species Colchicum. Up to now the formation of integumental tapetum in Liliaceae has been reported only by Poddubnaya-Arnoldi (Citation1976, Citation1982).
The hypostase and the integumentary obturator are common structures for Liliaceae according Davis (Citation1966) and Poddubnaya-Arnoldi (Citation1982), and are present in the studied species as well. The hypostase represents the border tissue between the nucellus, integument and chalaza, composed of cells with thickened walls. In medionucellate ovules, as was defined above the ovule in the Colchicum species, between the hypostase and the ES is a nucellar tissue that degenerates during seed formation (Shamrov Citation1999). The integumentary obturator represents the growth of epidermal cells of the inner integument and their withdrawal towards the micropyle (Poddubnaya-Arnoldi Citation1976).
Besides the hypostase and the integumentary obturator, in the nucellus of the studied Colchicum species we also observed the formation of a nucellar cap and podium. The nucellar cap is formed as a result of periclinal division of nucellar epidermal cells in the micropylar part of the ES. The podium is a glass-shaped structure formed in the chalazal part of the ES. Its upper border is located at the level of the antipodals and is divided from the ES by the parenchymal tissue of the nucellus. According to Shamrov (Citation1999) the podium is usually formed in the crassinucellate ovules with a long existing lateral area of the nucellus after fertilization. The present study showed that this structure can be defined as typical not only for the crassinucellate ovules but also for the medionucellate ones. The dividing of cells of the nucellar epidermis forming a two or more layered nucellar cap is reported also by Poddubnaya-Arnoldi (Citation1982), Dahlgren et al. (Citation1987) and Rudall (Citation1997) as characteristic in Colchicaceae.
The main function of all described specialized structures is the participation in the transport of substances in it: the hypostase – in the nucellus and integuments, the podium – in the lateral and apical areas of nucellus (Shamrov Citation1990, Shamrov and Аnisimova Citation1993; the nucellar cap – in the micropilar part of the nucellus, the integumental obturator – in the pollen tube penetrating in the ES (Poddubnaya-Arnoldi Citation1976). Their presence in the ovule of the studied species provides indication of the developing ES and is a sign of high specialization of the female generative sphere that in turn guarantees the successful development of the female gametophyte, leading to formation of viable seeds.
The literature data on endosperm development in Liliaceae show that it is cellular or helobial (Davis Citation1966; Poddubnaya-Arnoldi Citation1982), and in Colchicaceae it is nuclear (Watson and Dawliz 1992, Nordenstam Citation1998). Our study confirms the presence of a free nuclear stage in the studied species.
The established Polygonum type of ES formation is reported as typical for the Colchicaceae family (Dahlgren et al. Citation1987; Nordenstam Citation1998; Watson and Dawliz 1992) and is known as a basal one for the angiosperms (Poddubnaya-Arnoldi Citation1976).
In all studies on species of Liliaceae carried out until now, hooked synergids with filiform apparatus have been reported (Eunus Citation1950; Davis Citation1966; Poddubnaya-Arnoldi Citation1982), but in the studied species of Colchicum this phenomenon was not found.
The observed formation of an additional embryo in C. diampolis from one of the synergids was reported for the family Liliaceae by Davis (Citation1966) and Poddubnaya-Arnoldi (Citation1982). For the genus Colchicum the formation of an additional embryo is observed for the first time. For this genus a nucellar embryony was described (Dahlgren et al. Citation1987).
5. Conclusion
As a result of the present study the following embryological characteristics of genus Colchicum were determined:
Male generative sphere: tetrasporangiate anthers; monocotyledonous type of anther wall formation; five layered wall, consisting of epidermis, fibrous endothecium, two middle layers and glandular tapetum; successive microsporogenesis; two celled mature pollen.
Female generative sphere: trilocular ovary, anatropous, bitegmic, medionucellate ovule; unicellular archesporium; polygonum-type ES formation; Asterad-type embryogenesis; formation of podium, hypostase, nucellar cap and integumental tapetum; nuclear endosperm; poliembryony – development of additional embryo from one of synergids.
The stability of the processes in the male and female generative spheres (normal formation of male and female gametophytes without deviations and degenerative processes) ensures the successful realization of the reproductive potential of the studied species.
The high plasticity of the female gametophyte of C. diampolis expressed in the formation of an additional embryo from one of the synergids besides the legitimate one increases the adaptivity of its populations.
The high pollen and embryo viability play an important role in maintaining the size and state of populations of the three studied species.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bancheva S. 2010a. Colchicum bivonae guss. In: Peev D, editor. Red data book of Bulgaria vol 1 plants and fungi. Sofia: Academy of Sciences & MoEW; p. 454.
- Bancheva S. 2010b. Colchicum diampolis Delip. et Ceschm. In: Peev D, editor. Red data book of Bulgaria vol 1 plants and fungi. Sofia: Academy of Sciences & MoEW; p. 215.
- Biodiversity Act. 2002. State Gazette №77/02.08.2002. Sofia.
- Boyé O, Brossi A. 1992. Tropolonic Colchicum alkaloids and allo congeners. In: Brossi A, Cordell GA, editors. The alkaloids. Vol. 41. New York: Academic Press; p. 125–174.
- Copeland LO, McDonald MB. 2001. Principles of seed science and technology. 4nd ed. Boston: Springer.
- Dahlgren RM, Clifford HT, Yeo PF. 1987. The families of monocotyledons: structure. Evol Taxon. 7(3):254.
- Davis G. 1966. Systematic embryology of the angiosperms. New York (London, Sydney): John Wiley & Sons, Inc.
- Eunus AM. 1950. Contribution to the embyology of the Liliales. IV. Gametopkytes of smilacina stellata. New Phytol. 49(2):269–273.
- Evansa MEK, Menges ES, Gordonc DR. 2003. Reproductive biology of three sympatric endangered plants endemic to Florida scrub. Biol Conserv. 111:235–246.
- Heslop-Harrison JS. 1992. Pollen capture adhesion and hydration. In: Cresti M, Tizzi A, editors. Sexual plant reproduction. Berlin: Springer; p. 81–88.
- Jung LS, Winter S, Eckstein RL, Kriechbaum M, Karrer G, Welk E, Elsasser M, Donath TW, Otte A. 2011. Colchicum autumnale L. Perspect Plant Ecol Evol Syst. 13:227–244.
- Katzung BG. 2004. Basic and clinical pharmacology. 9th ed. New York: Lange Medical Books, McGraw-Hill; p. 527.
- Moore RP. 1985. Handbook on tetrazolium testing. Zurich (Switzerland): International Seed Testing Association.
- Nordenstam B. 1998. Colchicaceae. In: Kubitzki, editor. The families and genera of vascular plants, Vol.III flowering plants. Monocotyledons liliaceae (except Orchidaceae). Springer, Verlag GmBdt Heidelberg; p. 175–185.
- Palser BF. 1975. The bases of angiosperm phylogeny: embryology. Ann Missouri Bot Gard. 62:621–646.
- Peters J. 2000. Tetrazolium testing handbook. In: Contribution №29 to the handbook on seed testing revised. The Association of Official Seed Analysts (AOSA), PMB Las Cruces, USA.
- Poddubnaya-Arnoldi VA. 1976. Cytoembryology of the angiosperms. Moskva: Nauka. (in Russian).
- Poddubnaya-Arnoldi VA. 1982. Characteristics of Angiosperms families according to cytoembryological features. Moskva: Nauka. p. 196–205. (in Russian).
- Rudall PJ. 1997. The nucellus and chalaza in monocotyledons: structure and schematics. Bot Rev. 63(2):140–181.
- Shamrov I. 1990. The ovule of Gentiana cruciata (Gentianaceae): structural and functional aspects of its development. Bot. Journ. 75(10): 1363–1379. (in Russian)
- Shamrov I. 1999. The ovule as a basis for seed reproduction of flowering plants: classification of structures. Bot Journ. 84(10):1–35. (in Russian).
- Shamrov I, Аnisimova G. 1993. Peculiarites of the process of ovule formation in Luzula pedemontana (Juncaceae). Bot Jurn. 78(12):24–44. (in Russian).
- Singh RJ. 2003. Plant cytogenetics. 2nd ed. Boca Raton: CRC Press.
- Sundara RS. 2000. Practical manual of plant anatomy and embryology. New Delhi: Anmol Publ. Pvt Ltd.
- Watson L, Dallwitz MJ. 1992. Asteraceae Dum. In: The families of flowering plants: descriptions, illustrations, identification and information retrieval. http://delta-intkey.com

