ABSTRACT
In the present study, the changes in the arrangement of microtubules (MTs) were identified in the pistil cells of female, gall and male flowers exposed to programmed cell death (PCD) in Ficus carica, by using fluorescence and confocal microscopy. The microtubule organization was analyzed by labeling the pistil cells with FITC (fluorescein-isothiocyanate) and we noticed that they essentially show similarity in three morphs of the flowers prior to and during PCD. The MTs in small vacuolated cells homogenously oriented in the cytoplasm but intensively around the nucleus and within the cell cortex. MTs lie continuously parallel to the cortex and they circumferentially lie around the nucleus. MTs in large vacuolated cells radiate from the nucleus to the cortex and they appear as lightly thick bundles between cortex and vacuoles. The few small microtubule aggregations are present among the thick bundles. The organization of the MTs changes with the start of the PCD in the pistil cells. Vacuolization is one of the factors affecting MT organization in the cells showing PCD. Consequently, our data suggest that PCD affects the organization of MTs in the pistil cells and they gradually lose regular and parallel arrangement, coordinately with the degree of PCD.
1. Introduction
In angiosperms, programmed cell death (PCD) is an important process to maintain species-specific reproductive strategy and it occurs in variety of cells related with sexual reproduction, such as sex organ abortion in unisexual flowers, style transmitting tissue, non-functional megaspores, synergids, antipodals, nucellar cells, anther tapetum and abortive pollen in male sterility (Smith et al. Citation1992; Smertenko et al. Citation2003; Vardar and Ünal Citation2011, Citation2012; Zhang and Yang Citation2014; Aytürk and Vardar; Citation2015). It has attracted much attention recently, as a defensive factor to remove mutated, infected or damaged cells.
Ficus carica (Moraceae) has a complex reproductive cycle involving three flower morphologies [long-styled female, short-styled female (gall), male] located in the syconium and shows a mutual symbiosis with its pollinator wasp Blastophaga psenes (Agaonidae). The mutualistic symbiotic association between the gall flowers of fig and its pollinator wasp has received attention from several aspects (Ramirez Citation1974; Grandi Citation1999; Armstrong Citation2011). This interaction may result in some responses in plant tissues such as cellular disorders leading to PCD (Giblin-Davis et al. Citation1995; Durme and Nowack Citation2016). Based on the results of our previous study, cellular disorders such as cytoplasm and vacuole collapse and degeneration, vacuolization, chromatin condensation and nuclear irregularities suggest that PCD takes place in the pistil tissue of gall flowers after oviposition (Aytürk Citation2016). Cytoplasmic changes in response to various internal and external stimuli are dependent on, and sometimes controlled by the cytoskeleton (Williamson Citation1993).
The roles of the cytoskeleton, consisting mainly of microtubules (MT) and microfilaments (MF), in plant biology are multifaceted (Zandomeni and Schopfer Citation1994; Geitmann et al. Citation2000; Takemoto et al. Citation2006; Borowiak et al. Citation2015). According to the results of Smertenko et al. (Citation2003) the organization of the cytoskeleton changes during developmental PCD in Picea abies embryos, and it is more obvious from the embryonal mass towards the distal end of the embryo suspensor. In highly vacuolated suspensor cells the microtubules completely degrade, followed by DNA fragmentation and vacuole collapse (Filonova et al. Citation2000a).
Although there are a number of studies on the reorganization of MTs related with PCD on animals (Ndozangue-Touriguine et al. Citation2008), there are limited detailed studies in plants (except Smertenko et al. Citation2003). In the light of these studies, we will first observe the orientation of the microtubules in the pistil cells prior to PCD and thereafter, we will compare the microtubular organization in the course of PCD at the same tissue cells.
2. Material and methods
2.1. Plant materials
The female, gall and male flowers of F. carica at various stages of development were collected from Göztepe Campus of Marmara University, from October 2010 to June 2014.
The occurrence of PCD as confirmed by DNA fragmentation and the organization of microtubules in the pistil cells was analyzed by fluorescence and confocal microscopy with the following procedures.
2.2. Microscopic examination
For fluorescence microscopy, the flowers were fixed in FAA (ethanol: 50 ml, glacial acetic acid: 5 ml, formaldehyde (37–40%): 10 ml, distilled water: 35 ml) and kept in a vacuum for 1 h at 25°C. Then, the examples were rinsed in buffer1 (37% paraformaldehyde) for one night at + 4°C and embedded in paraffin. The longitudinal sections of the flowers were cut 2–3 μm in thickness by a Leica RM2235 rotation microtome (Heidelberger, [Nussloch GmbH], Germany) and placed on poly-L-lysine-coated slides. They were deparaffinized prior to the immunolabeling procedure.
For confocal microscopy the flowers were fixed in glutaraldehyde [2.5% glutaraldehyde in 0.025 M phosphate buffer (pH 7.2)]. After embedding in epon, the longitudinal semithin sections were cut into 1 μm by Leica 705,902 ultracut and placed on poly-L-lysine-coated slides, as adapted from Vardar and Ünal (Citation2012). After removal of epon, the sections were immunolabeled.
2.3. Determination of DNA fragmentation
The histological sections of each pistil of female and male flowers were stained by DAPI (4–6-diamidino-2-fenilindol) (1 µg ml–1) (Lloyd and Traas Citation1988) for the diagnosis of the nuclear morphology. The TUNEL (terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling) technique is the labeling of free 3ʹOH termini with modified nucleotides in an enzymatic reaction that identifies the DNA strand breaks. For this reaction, sections were pasted to poly-L-lysine coated slides and incubated in reagents from ApopTag® Plus In Situ Apoptosis Fluorescein Detection Kit (Chemicon, Temecula, [CA], USA)), following the manufacturer’s instructions. The negative controls were labeled in parallel except for the absence of the TdT.
2.4. Microtubule immunostaining
The sections were rinsed with buffer 1 for 30 min and exposed to one of the following wall-digesting enzymes, each dissolved in 0.4 M mannitol: 0.5% cellulase (Sigma-Aldrich, C1184, St Louis, [MO], USA) for 25 min; 2% driselase (Sigma D8037) for 15 min; 1% cellulysin (Sigma EMD 219,466) for 15 min respectively. After the treatment with each enzyme the specimens were rinsed in buffer 1 for 5 min. The specimens were rinsed with chilled methanol for 10 min and then rinsed with buffer 2 (1% Triton X, 0.1 M PIPES, 2mM EGTA, 1 mM MgSO4 and 0.4 M mannitol pH6.9) for 30 min. Later, the sections were incubated in anti-tubulin antibody [1 µg IgG/20 µl buffer 2, (Sigma T-5168)] for one hour at 37°C, in a dark and humid environment. They were then rinsed for with buffer 2 for 1 min. Following this, the specimens were incubated in fluorescein goat anti-rabbit IgG [10 µg IgG/240 µl buffer 2 (Boehringer, Mannheim, Germany, 821,462)] at 37°C, in a dark and humid environment for 30 min. They were rinsed for three times with buffer 2 for 2 min.
Controls included use of a rabbit serum, which gave no staining of the microtubule which used at a 1:10 dilution in buffer 2. Lastly, DABCO [1,4-diazabicyclo (2,2,2) octane] (2 DABCO/98 buffer 2) was dropped to prevent fading and visualized by fluorescent and confocal microscopy.
Samples for DAPI and TUNEL stained samples were examined were examined with a Leica DM LB2 fluorescence and Leica DM 5500 CS confocal microscope (excitation: 330–385 nm for DAPI and 460–490 nm for TUNEL). For fluorescence microscopy the organization of microtubules was monitored using an DM LB2 model Olympus BX-51 microscope (Argenit, [Istanbul], Turkey) with excitation and barrier filters optimized for FITC fluorescence (excitation 450–490 nm, emission 420 nm). Images were collected using a digital camera and processed using image analysis software (Kameram, Argenit, [Istanbul], Turkey). For confocal microscopy, labeled flower specimens were viewed with an Leica DM 5500 CS confocal laser scanning system and a 40 and 60 × oil-immersion objective lens.
3. Results
3.1. PCD in the flowers
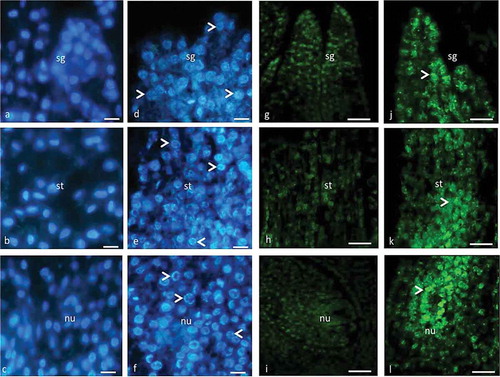
The pistil development was investigated starting from initiation to the end of the tissue disruption in the female and male flowers. In female flowers, the stigma, style and nucellar tissue cells show cellular disorders after pollination. In male flowers, the entire pistil tissue cells show the same disorders. In our previous study, we found out that cellular disorders occur following loading of wasp eggs to the pistil tissue of the gall flower (Aytürk Citation2016). The cellular disorders include vacuolization, chromatin condensation, cytoplasmic and vacuolar collapse, which are the common hallmarks of PCD in three morphs of flowers. DAPI assay clearly showed the condensed chromatin at the nuclear periphery of the pistil cells in the early stage of PCD and complete disruption at the advanced stages. DNA fragmentation was obvious after the application of TUNEL assay. Prior to PCD, the elliptical nuclei of cells emit bright blue fluorescence and the chromatin is evenly dispersed over the nucleus. With the onset of PCD the chromatin, instead of evenly spreading, starts to condense into some granules at the nuclear periphery. At the advanced stage, the chromatin condensation progresses with emitting increasingly intensive fluorescence. TUNEL staining is negative prior to PCD, whereas it sharply stains the nuclei with the start of PCD. PCD first starts at the style then appears in stigma and finally at the nucellar tissue. TUNEL is intensively positive at the advanced stage of the PCD and it ends shortly after. Following this stage only autofluorescence is visible in these tissues ( and ).
Figure 1. Stigma (a, d, g, j), style (b, e, h, k) and nucellar tissue (c, f, i, l) of female flower with DAPI (a–f) and TUNEL (g–l) assay. (a–c) Prior to PCD, chromatin is evenly distributed in the nuclei. (g–i) Prior to PCD, TUNEL is negative. (d–f) Condensed chromatin (arrow) in nuclei in advanced stage of PCD. (j–l) TUNEL is positive (arrow) in advanced stage of PCD. sg, stigma; st, style; nu, nucellus. Scale 10 µm (a–f) and 50 µm (g–l).
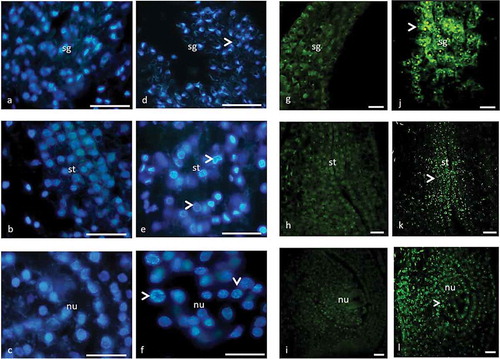
Figure 2. Stigma (a, d, g, j), style (b, e, h, k) and nucellar tissue (c, f, i, l) of male flower with DAPI (a–f) and TUNEL (g–l) assay. (a–c) Prior to PCD, chromatin is evenly distributed in the nuclei. (g–i) Prior to PCD, TUNEL is negative. (d–f) Condensed chromatin (arrow) in nuclei in advanced stage of PCD. (j–l) TUNEL is positive (arrow) in advanced stage of PCD. sg, stigma; st, style; nu, nucellus. Scale 10 µm (a–f) and 50 µm (g–l).
3.2. The microtubule organization in the pistil cells prior to PCD
The structural and the developmental features in all three flowers show similarity in the early stages and with the onset of PCD the differences start to become obvious. Based on these findings, we scrutinize microtubule organization in the pistil cells before they undergo PCD, and in the course of the PCD process.
The pistil primordial cells with small vacuoles are located just below the epidermis and in older cells with large vacuoles are located deep down. The MTs are homogenously oriented all over the cytoplasm but intensively around the nucleus and within the cell cortex ()). MTs lie continuously parallel to the cortex as a thin layer and they circumferentially lie around the nucleus. However, MTs in large vacuolated cells radiate from the nucleus to the cortex ()). It should be noted that the MTs give weak fluorescence in the primordial cells in contrast to the cells undergoing PCD.
Figure 3. The organization of immunofluorescently labeled microtubules in the pistil cells of female, gall and male flowers prior to programmed cell death visualized by fluorescence microscopy (c) and laser confocal microscopy (a, b, d). The arrowheads show fluorescence signal on the cortical region, around the nuclei and vacuoles. The double arrowheads show thick fluorescent aggregates. Scale 10 μm.
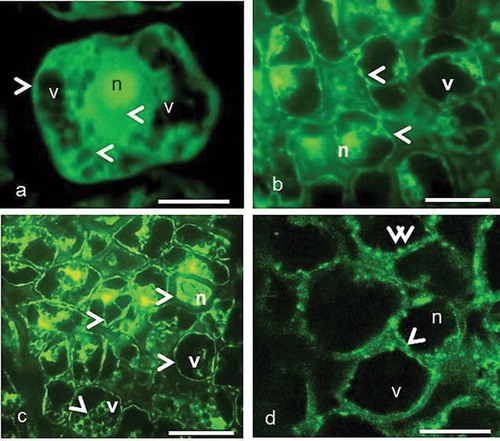
When the ovule differentiates in the ovary, the style starts to elongate in female, male and gall flowers. In contrast to primordial cells, the entire pistil tissue is composed of large vacuolated cells. Vacuolization affects the orientation of MTs. The nucleus is pushed to one side of the cell and MTs are located around the nucleus. MTs circumferentially surround the vacuoles and nucleus ()) and they appear as thick bundles between cortex and vacuoles ()).
MTs exhibit similar organization in the pistil cells of female and gall flowers up to stage of mature embryo sac and they continue to form thick bundles in cell cortex. The small microtubule aggregations were present among the thick bundles ()). In male flowers, the ovule does not differentiate in the pistil and the cells start to undergo PCD, in contrast to female and gall flowers, where normal development continues.
3.3. Microtubule reorganization in the pistil cells in the course of PCD
Immunocytochemical staining of tubulin revealed that PCD causes reorganization in the MT cytoskeleton in the cells of style and nucellus in response to the oviposition in gall flowers, and in the same tissues of pollinated female flowers. In other words, the MT orientation varies by the occurrence of PCD and the reorganization of MTs takes place in the same way in female, gall and male flowers. With the start of PCD, MTs lie discontinuously parallel to the plasma membrane and aggregations become more obvious around the nuclei, and particularly at the cells near stylar canal where the ovipositor penetrates in a gall flower ()). Vacuolization increases with the progress of PCD and MTs bundles become thicker in the cell cortex. They are irregularly aligned around the nucleus ()).
Figure 4. The organization of immunofluorescently labeled microtubules in the pistil cells of female, gall and male flowers in the course of programmed cell death visualized by laser confocal microscopy. (a–c) The arrowheads show fluorescence signal on the cortical region, around the nuclei and vacuoles. The double arrowheads show thick fluorescent aggregates. (d) The randomly organized cortical MT network. n, nucleus; v, vacuole. Scale 10 μm.
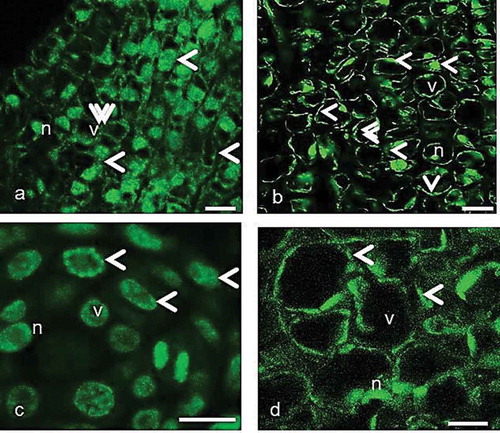
In the advanced stages, MTs gradually lose regular and parallel organization and become randomly oriented in the pistil cells of female, gall and male flowers. This situation proceeds in the same way by the end of development (). In the gall flowers, when a mature wasp appeared in the ovule, the stylar and nucellar tissues completely break down and the MTs became invisible.
In the male flowers, the entire pistil tissue cells undergo PCD and the stigma and style completely arrested and the ovary remains discernable.
4. Discussion
In the present paper, using fluorescence and confocal microscopy the organization of microtubules was identified in the pistil cells prior to and during the course of PCD in three flowers morphs of Ficus carica, showing mutualistic symbiosis with a wasp.
We have confirmed by using DAPI and TUNEL techniques that the pistil cells of female (pistillate) and male flowers (staminate) in F. carica showed characteristics of PCD including DNA fragmentation, chromatin condensation, nuclear disorders. Moreover, in our previous study we have identified PCD in the pistil tissue cells of the gall flowers serving as host for a specific pollinator wasp Blastophaga psenes after oviposition (Aytürk Citation2016). While PCD occurs in the stigma, stylar transmitting tissue and nucellus of the female flowers after pollination, it takes place in the same tissues of the gall flowers after oviposition. In the male flowers the development of pistil is arrested as a result of PCD. The occurrence of PCD in the transmitting tissue is usual for angiosperms (Herrero Citation1992; Gunawardena and Gaolathe Citation2015). This process also takes place as a result of various stress responses including innate immunity against a pathogen attack (Pennell and Lamb Citation1997). Microtubular cytoskeleton reorganizations were observed in the early symbiotic steps in the Medicago/Rhizobium meliloti symbiotic interaction (Timmers et al. Citation1999). They announced that the cytoskeleton is involved in the early symbiotic steps. Our PCD results on the style of female and gall flowers show analogies with those of Geitmann and Emons (Citation2000) and Serrano et al. (Citation2010). According to Wu and Cheung (Citation2000) some deteriorations in the stigmatic and stylar tissues were interpreted as PCD with pollination considered an inducer during pollen–pistil interaction.
The microtubules in the pistil tissue cells of three morphs of fig flowers show similar organization prior to and in the course of PCD. In the cells prior to PCD, the MTs lie in parallel arrangement to the cell cortex as a continuous thin layer and MTs network randomly organizes from the nucleus to the cytoplasm. In addition, MTs appear homogeneously around the vacuoles in the cytoplasm. With the start of PCD, changes in MT organization become more obvious. Fluorescent MTs, previously at low density, increase in the advanced stages around the nucleus and vacuole and in the cell cortex. The increment of vacuolization associated with PCD affects the organization of microtubule cytoskeleton. The degree of the changes coordinately increases with the progression of the PCD. In plants, cellular morphological changes are governed by the cytoskeleton (Franklin-Tong and Gourlay Citation2008) and vacuolization (Higaki et al. Citation2011). Rapid development of live cell imaging techniques has recently revealed novel aspects of the dynamics of these intracellular structures (Kumagai et al. Citation2001; Kutsuna and Hasezawa Citation2002; Sano et al. Citation2005; Higaki et al. Citation2007, Citation2008). According to Filonova et al. (Citation2000a), provacuoles turn into the autolytic vacuoles which destroy the cytoplasmic content in PCD. So, it is possible that the PCD causesthe re-orientation and the subsequent disorganization of MTs (Andrei et al. Citation2003). In the pistil cells of F. carica, the MT aggregates occur in the mentioned sites associated with the PCD. Moreover, the thick MT bundles appear in the cells, with increasing breakdown caused by PCD. These findings agree with the studies of Andrei et al. (Citation2003) and Filonova et al. (Citation2000b). Filonova et al. (Citation2000a) observed complete degradation of microtubules in highly vacuolated suspensor cells, followed by DNA fragmentation and vacuole collapse. Smertenko et al. (Citation2003) drew a schematic model for the re-organization of the cytoskeleton in the course of PCD associated with embryonic pattern formation in Picea abies. These findings indicate that the organization of microtubules is an effective factor for PCD during embryogenesis. In the advanced stage of PCD in the pistil cells of F. carica, MTs progressively lose their parallel organization leading to a random organization. Moreover, in tobacco, PCD was triggered by application of oomycete elicitor cryptogein protein and MTs disintegrate speedily and the number of microtubular aggregates and thick bundles increase (Binet et al. Citation2001; Takemoto et al. Citation2003, Citation2006; Shan and Godwin Citation2004). Similarly, the cytoskeletal organization changes in Linum usitatissimum cells showing hypersensitive cell death by the attraction with the fungus Melampsora lini (Kobayashi et al. Citation1994; Skalamera and Heath Citation1998).
In conclusion, the microtubule network reorganizes in the pistil cells of F. carica undergoing PCD. This result has importance in developmental plant biology but needs further study to elucidate of PCD signal transduction to the cytoskeleton.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Andrei PS, Peter VB, Lada HF, Sara VA, Patrick JH. 2003. Re-organisation of the cytoskeleton during developmental programmed cell death in Picea abies embryos. Plant J. 33:813–824.
- Armstrong WP (2011). Sex Determination and Life Cycle Of Ficus carica. https://www2.palomar.edu/users/warmstrong/pljun99b.htm.
- Aytürk Ö (2016). Dioik Ficus carica L. (İncir)’de Dişi, Gal ve Erkek Çiçek Gelişiminin Mikroskobik ve Moleküler Yöntemler ile Karşılaştırılması Ficus carica L. (Fig) (Comparison of Female, Gall and Male Flower Development with Microscopic and Molecular Tecniques in dioecious Ficus carica L. (Fig)) [unpublished PhD thesis]. İstanbul-Turkey: Marmara Üniversitesi.
- Ö A, Vardar F. 2015. Aluminum-induced caspase like activities in some Gramineae species. Adv Food Sci. 2:1–6.
- Binet MN, Humbert C, Lecourieux D, Vantard M, Pugin A. 2001. Disruption of microtubular cytoskeleton induced by cryptogein, an elicitor of hypersensitive response in tobacco cells. Plant Physiol. 125:564–572.
- Borowiak M, Nahaboo W, Reynders M, Nekolla K, Jalinot P, Hasserodt J, Rehberg M, Delattre M, Zahler S, Vollmar A, et al. 2015. Photoswitchable inhibitors of microtubule dynamics optically control mitosis and cell death. Cell. 162:403–411.
- Durme MV, Nowack MK. 2016. Mechanisms of developmentally controlled cell death in plants. Curr Opin Plant Biol. 29:29–37.
- Filonova LH, Bozhkov PV, Brukhin VB, Daniel G, Zhivotovsky B, Von Arnold S. 2000a. Two waves of programmed cell death occur during formation and development of somatic embryos in the gymnosperm, Norway spruce. J Cell Sci. 113:4399–4411.
- Filonova LH, Bozhkov PV, Von Arnold S. 2000b. Developmental pathway of somatic embryogenesis in Picea abies as revealed by time-lapse tracking. J Exp Bot. 51:249–264.
- Franklin-Tong VE, Gourlay CW. 2008. A role for actin in regulating apoptosis/programmed cell death: evidence spanning yeast, plants and animals. Biochem J. 413:389–404.
- Geitmann A, Emons AM. 2000. The cytoskeleton in plant and fungal cell tip growth. J Microsc. 198:218–245.
- Geitmann A, Snowman BN, Emons AMC, Franklin-Tong VE. 2000. Alterations in the actin cytoskeleton of pollen tubes are induced by the self-incompatibility reaction in Papaver rhoeas. Plant Cell. 12:1239–1251.
- Giblin-Davis RM, Williams D, Hewlett TE, Dickson DW. 1995. Development and host attachment studies using Pasteuria from Belonolaimus longicaudatus from Florida. J Nematol. 27:500.
- Grandi G. 1999. Gli insetti dei caprifichi. Riv Biol. 5:69–90.
- Gunawardena A, Gaolathe R. 2015. Programmed cell death: genes involved in signaling, regulation, and execution in plants and animals. Botany. 93:193–210.
- Herrero M. 1992. Mechanisms in the pistil that regulate gametophyte population in peach (Prunus persica). In: Ottaviano E, Mulcahy DL, Sari Gorla M, Bergamini Mulcahy G, editors. Angiosperm pollen and ovule. 1st ed. New York: Springer; p. 377–381.
- Higaki T, Goh T, Hayashi T. 2007. Elicitor-induced cytoskeletal rearrangement relates to vacuolar dynamics and execution of cell death: in vivo imaging of hypersensitive cell death in tobacco BY-2 cells. Plant Cell Physiol. 48:1414–1425.
- Higaki T, Kadota Y, Goh T. 2008. Vacuolar and cytoskeletal dynamics during elicitorinduced programmed cell death in tobacco BY-2 cells. Plant Signal Behav. 3:700–703.
- Higaki T, Kurusu T, Hasezawa S. 2011. Dynamic intracellular reorganization of cytoskeletons and the vacuole in defense responses and hypersensitive cell death in plants. J Plant Res. 124:315–324.
- Kobayashi I, Kobayashi Y, Hardham AR. 1994. Dynamic reorganization of microtubules and microfilaments in flax cells during the resistance response to flax rust infection. Planta. 195:237–247.
- Kumagai F, Yoneda A, Tomida T. 2001. Fate of nascent microtubules organized at the M/G1 interface, as visualized by synchronized tobacco BY-2 cells stably expressing GFP tubulin: time-sequence observations of the reorganization of cortical microtubules in living plant cells. Plant Cell Physiol. 42:723–732.
- Kutsuna N, Hasezawa S. 2002. Dynamic organization of vacuolar and microtubule structures during cell cycle progression in synchronized tobacco BY-2 cells. Plant Cell Physiol. 43:965–973.
- Lloyd CW, Traas JA. 1988. The role of f-actin in determining the division plane of carrot suspension cells. Drug Studies. Development. 102:211–222.
- Ndozangue-Touriguine O, Hamelin J, Bre´Ard J. 2008. Cytoskeleton and apoptosis. Biochem Pharmacol. 76:11–18.
- Pennell RI, Lamb C. 1997. Programmed cell death in plants. Plant Cell. 9:1157–1168.
- Ramirez W. 1974. Coevolution of ficus and agaonidae. Ann Missouri Bot Gard. 61:770–780.
- Sano T, Higaki T, Oda Y. 2005. Appearance of actin microfi lament ‘twin peaks’ in mitosis and their function in cell plate formation, as visualized in tobacco BY-2 cells expressing GFP-fi mbrin. Plant J. 44:595–605.
- Serrano I, Pelliccione S, Olmedilla A. 2010. Programmed-cell-death hallmarks in incompatible pollenand papillar stigma cells of Olea europaea L. Under Free Pollination Plant Cell Rep. 29:561–572.
- Shan XC, Goodwin PH. 2004. Monitoring host nuclear migration and degradation with green fluorescent protein during compatible and incompatible interactions of Nicotiana tabacum with Colletotrichum species. J Phytopathol. 152:454–460.
- Skalamera D, Heath MC. 1998. Changes in the cytoskeleton accompanying infection-induced nuclear movements and the hypersensitive response in plant cells invaded by rust fungi. Plant J. 16:191–200.
- Smertenko AP, Bozhkov PV, Filonova LH, Arnold SV, Hussey PJ. 2003. Re-organization of the cytoskeleton during developmental programmed cell death in Picea abies embryos. Plant J. 33:813–824.
- Smith MT, Saks Y, Van Staden J. 1992. Ultrastructural changes in the petals of senescing flowers of Dianthus caryophyllus L. Ann Bot. 69:277–285.
- Takemoto D, Jones DA, Hardham AR. 2003. GFP-tagging of cell components reveals the dynamics of subcellular re-organization in response to infection of Arabidopsis by oomycete pathogens. Plant J. 33:775–792.
- Takemoto D, Jones DA, Hardham AR. 2006. Re-organization of the cytoskeleton and endoplasmic reticulum in the Arabidopsis pen1-1 mutant inoculated with the non-adapted powdery mildew pathogen, Blumeria graminis f. Sp Hordei Mol Plant Pathol. 7:553–563.
- Timmers AC, Auriac MC, Truche G. 1999. Refined analysis of early symbiotic steps of the Rhizobium-Medicago interaction in relationship with microtubular cytoskeleton rearrangements. Development. 126:3617–3628.
- Vardar F, Ünal M. 2011. Cytochemical and ultrastructural observations of anthers and pollen grains in Lathyrus undulatus Boiss. Acta Bot Croat. 70:53–64.
- Vardar F, Ünal M. 2012. Ultrastructural aspects and programmed cell death in the tapetal cells of Lathyrus undulatus Boiss. Acta Bot Hung. 63:56–70.
- Williamson RE. 1993. Organelle movements. Annu Rev Plant Physiol. 44:181–202.
- Wu HM, Cheung AY. 2000. Programmed cell death in plant reproduction. Development. 44:267–281.
- Zandomeni K, Schopfer P. 1994. Mechanosensory microtubule reorientation in the epidermis of maize coleoptiles subjected to bending stress. Protoplasma. 182:96–101.
- Zhang D, Yang L. 2014. Specification of tapetum and microsporocyte cells within the anther. Curr Opini Plant Biol. 17:49–55.

