Abstract
Aim
This paper aimed to answer how psychometric methods based on neurotypical populations can serve as valid instruments in the assessment and diagnosis of intellectual disability in individuals with atypical development. The genetic, structural, and functional features of CHARGE make it uniquely suited to address this question.
Method
A Norwegian population of individuals with CHARGE (N = 35) underwent assessment procedures according to DSM-5 guidelines for the evaluation of an intellectual disability diagnosis. Results from cognitive testing (Wechsler Intelligence Scales) and parental evaluation of adaptive skills (Vineland Adaptive Behavioral Scale) were obtained and compared to their respective norm samples to explore any methodological inconsistencies.
Result
Significant differences emerged between the participants and the norm samples. Global cognition obtained from Wechsler revealed a bimodal distribution, suggesting a two-group sample, with the youngest children forming their own subgroup. Comparisons of the different age-groups’ performances demonstrated the lowest results among the preschoolers while the adults scored the highest. The global adaptive behavior score turned out significantly lower than the performance-based scores, thereby deflating the overall estimate of global intellectual abilities.
Conclusion
For individuals with CHARGE, the effect of the atypicality seemed most apparent during early childhood, stabilizing and subsiding towards adulthood. The test results’ interpretability was weakest for the preschoolers progressively increasing until peaking in adulthood, emphasizing the importance of delaying the assessment and diagnosis of intellectual disability. Because of several validity issues connected to the observation-based measure, complementary testing should precede clinical evaluations when possible in the diagnostics of individuals with CHARGE.
Introduction
This study rests on the assumption that children and adolescents with atypical development are subject to underestimation of intellectual ability because of incongruence between their sensorimotor challenges and test methodology. While norm-referenced assessments ideally should ascertain the validity of clinical diagnoses [Citation1], two opposing premises threaten this certainty in the assessment and diagnosis of intellectual disability [Citation2]. First, most test procedures and norms in these evaluations derive from neurotypical populations’ performances and behavioral characteristics [Citation3–7]. Second, those referred for these assessments have deviated in some respect from the expected (i.e. the norm), often because of a neurodevelopmental disorder. Conducting differential diagnosis implies a combination of these two opposites, which may compromise diagnostic validity.
According to the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) [Citation2], intellectual disability is defined as ‘a disorder with onset during the developmental period that includes both intellectual and adaptive functioning deficits in conceptual, social, and practical domains’ (p. 33). The diagnosis rests on fulfilling three criteria (A, B, and C). Criterion A involves the individual’s degree of global cognitive deficit, which implies an estimate below 70 (cutoff score) obtained on an established intelligence scale [Citation5–7]. Criterion B specifies the degree of severity found in A by evaluating challenges within adaptive behavior. Often involved is a norm-based global estimate of adaptive abilities according to a standardized observational measure, such as Vineland Adaptive Behavioral Scale [Citation4]. Like criterion A, the estimate should be below 70 to fulfill criterion B. The last criterion (C) implies that challenges identified in A and B must appear before adulthood [Citation2].
Meanwhile, the criteria for diagnosing intellectual disability may rest on incompatible methodological prerequisites for children and adolescents deviating from the norm [Citation8]. Support or rejection of such assumption builds on findings from investigations of individuals with atypical development according to the established neurotypical principles [Citation9]. Two reasons make CHARGE syndrome (OMIM 214800) (CHARGE for short) appropriate for this purpose. First, knowledge of intellectual strengths and challenges related to CHARGE, including intellectual disability, remains uncertain. Comparisons of research addressing intellectual functioning in CHARGE reveal considerable variation [Citation10]. This lack of consensus is likely related to the lack of acknowledged standardised psychometric methodology and population-based designs. Despite methodological shortcomings, the incidence of intellectual disability within the samples is often reported and appears to vary between 18% and 100% (for an overview, see ) [Citation10].
Table 1. Summary of studies addressing cognitive and behavioral functioning of individuals with CHARGE adapted from the review by Thomas et al. [Citation10].
Second, despite being rare [Citation52], CHARGE shares many features with other neurodevelopmental disorders and congenital conditions [Citation53], which makes it uniquely suited for cross-condition comparisons and knowledge exchange. Particularly useful is the potential transference of knowledge to other low-frequent congenital disorders affecting the auditory and visual senses [Citation54].
CHARGE syndrome is typically associated with mutations in the chromodomain helicase DNA-binding protein-7 (CHD7) (OMIM 608892), which are assumed to cause its characteristic complex phenotype heterogeneity [Citation55]. Affected audiovestibular functions constitute a significant feature in CHARGE [Citation56]. Unilateral or bilateral auditory impairment arises from anomalies within the semi-circular canals, ultimately disturbing communication processes and language acquisition [Citation29]. Coloboma is also prominent in CHARGE, functionally manifested by visual field defects and stereopsis problems and often accompanied by reduced visual acuity [Citation57]. These visual deficits restrict the child’s access to critical environmental stimuli, impacting mental processes. For about 50% of the CHARGE population, combined impairments of primary senses are so severe that they constitute a deafblind condition [Citation58]. Deafblindness is not a medical diagnosis but a functional description of the co-occurring challenges within social life, access to information, and mobility unfolding when the less affected sense fails to compensate for the deficits of the other [Citation59].
In addition to these major features and several other characteristics (https://www.chargesyndrome.org), intermediate bidirectional mechanisms between different levels of inference give rise to other psychomotoric dysfunctions [Citation60]. For example, anomalies within the vestibular apparatus influence the child’s balance and motoric self-efficacy, overall mobility, and dexterity [Citation61]. One clear manifestation of this effect is the delayed age of onset of independent walking, which for children with CHARGE is, on average, around three years [Citation62]. The delay is considerably later than their neurotypical peers. It is also later than children with severe visual impairment, who, on average, walk independently at around 20 months [Citation63].
By investigating the Norwegian CHARGE population, this study aimed to test the hypothesis stating that individuals with atypical development are at increased risk of underestimation of intellectual ability and, thereby, obtain false positive diagnoses of intellectual disability because of incongruences between the features of their primary condition, and the methods and principles used in the assessment and diagnosis of intellectual disability.
Methods
Study design and cohort
A cross-sectional national population of individuals with CHARGE underwent assessments following the diagnostic guidelines of intellectual disability (DSM-5, [Citation2]). The findings from measures related to criteria A and B constituted the study’s main dependent variables, and the norm sample of the different instruments served as independent variables.
Recruitment occurred in 2020, with a confirmed CHARGE diagnosis as the only inclusion criterion. No national registry of individuals with CHARGE exists in Norway, which renders the national prevalence uncertain. However, the administrative prevalence approximated 40 in 2017 [Citation64]. Several strategies were employed to locate as many participants as possible, including contacting medical, social, and educational services nationwide and announcements on social media. The final sample included 35 Norwegians with CHARGE.
Data and procedures
The data were collected between 2020 and 2022 and consisted of four phases: (i) primary (first contact with parents and participants, planning of the subsequent phases), (ii) pre-assessment (getting acquainted before testing), (iii) assessment (conducting cognitive testing and interviews), and (iv) post-assessment (follow-up with both oral and written dissemination of findings). The first author collected all the data, including cognitive testing, interviews, and other relevant information.
Parameters of relevance to the assessment outcomes included participants’ auditory and visual function, age of onset of independent walking, and language and communication (). Besides one participant, who demonstrated auditory processing disorder during the assessment, all had confirmed unilateral (n = 15) or bilateral (n = 19) hearing loss. About two-thirds of the participants had confirmed coloboma. Thirteen participants had no confirmed visual challenges beyond that corrected by glasses, and two had blindness. While 10 participants had a mild reduction of visual acuity, and 8 had moderate, 18 had severe functional deficits due to their visual challenges. Eighteen of the 35 participants had deafblindness. Five of these 18 had cerebral palsy and epilepsy, medical conditions beyond those explicitly in the CHARGE criteria.
Table 2. Selected demographic, sensory, and developmental information of the participants (N = 35).
Seventeen participants used spoken language, of whom five needed sign support: nine participants used Norwegian Sign Language as their first language, and five used tactile sign language as their primary mode of communication: the communication mode of 4 toddlers was still undecided. The mean onset of independent walking was three years and seven months, and for speaking or signing three years and two months. About two-thirds received hearing aids before they turned 2.
The participants’ global cognition (criterion A) was estimated by the latest editions of the Wechsler Intelligence Scales for preschoolers (WPPSI-IV), children and adolescents (WISC-V), and adults (WAIS-IV) [Citation5–7] available in Norway. One toddler was below the age limits and evaluated with the third version of the Bayley Scales of Infant and Toddler Development (Bayley-III) [Citation3]. The cognitive Quotient (CQ in the following) was a common denominator for overall cognition. Criterion B rested on the parental semi-structured interview Vineland Adaptive Behavior Scale (2nd edition) [Citation4], which gives a global adaptive behavioral estimate (GAI) for individuals between age 2.0 and 21.11 (according to the Norwegian version). The Communication, Daily Living Skills, and Socialization indices were summarized to produce the GAI. The domain of Motor Skills is part of the GAI estimate for children up to age 6.
According to the Individuals with Disabilities Education Act (2004) (https://sites.ed.gov/idea), the participants qualified for adapted test conditions due to their sensory and motor impairments. In line with established categorization, these adaptations, denoted accommodations, divide into presentation, response, setting, timing, and equipment/devices [Citation68] and are evaluated after the Standards for education and psychological measurement [Citation69]. The Standards involve the comparability of scores obtained from accommodated testing with standardized test results (i.e. if the accommodated test is the same test as the original) [Citation70]. One source of validity is the principle of the differential boost, which states that individuals with disabilities should gain more from the accommodations than those without deficits [Citation71,Citation72].
The present study’s accommodations and validation evaluations [Citation68] are presented in . Besides test administration in sign language, the comparability between accommodated and standardized test items remained relatively unaffected. However, administrating test items in sign language entailed validity concerns and needed additional analyses. Correct translation and interpretation require bilingual knowledge and insights into the sign-language culture [Citation73]. Comparability evaluation also rests on the clinician’s knowledge of the developmental neurocognitive consequences of growing up with auditory impairment [Citation75]. Accordingly, ‘…deaf children are not simply hearing children who cannot hear’ ([Citation74], p. 136).
Table 3. Categories of accommodations according to the review by Sireci and O’Riordan [Citation68] transferred to the present study to evaluate the comparability of scores obtained from test-items before and after any accommodations.
Several measures in the present study addressed the comparability and validity of testing involving sign language. The participants’ preferred language modality had priority in all the assessments, and their regular communication partners adjoined the test sessions to safeguard communication. Because different grammatical rules apply to Norwegian sign language compared to speech, the wording of the test instructions and questions had to be changed. Each response was evaluated thoroughly with a fluent signer to ensure the difficulty level remained unaffected. The changed wording was considered a prerequisite for participating and not an advantage. As such, sign language also involved differential boosting. Despite the complexity of risks connected to test administration in sign language, test validity was considered ascertained by the precautions taken.
Five of the 35 participants needed adaptations beyond the provided accommodations. These more comprehensive changes in test conditions denoted modifications [Citation69], involve a heightened risk of compromising test validity [Citation76]. The modifications included longitudinal follow-up before formal assessment to establish prerequisite skills needed to succeed on performance-based measures as these were lacking [Citation77]. None of them could complete the age-relevant version of Wechsler. The 5 participants also differed from the others regarding symptom load by presenting additional medical complications, such as cerebral palsy and epilepsy, along with the severe degree of combined sensory impairments (i.e. severe to profound hearing loss combined with blindness). Therefore, results from both testing and the parental reports were excluded from the data set to avoid contamination and jeopardize psychometric validity.
The study was approved by the Norwegian Regional Committees for Medical and Health Research Ethics (#2020/43412).
Statistical analysis
All measures have identical mathematical properties with a mean of 100 and a standard deviation of 15, making cross-comparison of test findings possible.
Normality tests were satisfactory for both dependent variables (CQ and GAI) with non-significant Shapiro-Wilk, and skewness and kurtosis within the requirements ( and ). Pearson correlation explored the associations between the dependent variables, and paired sample t-tests addressed the differences.
Figure 1. Distribution of the participants’ performance-based assessment after excluding the 5 who needed modified test conditions (n = 30). Despite skewing to the right, the distribution was considered normal according to the tests of normality. The distribution indicated a bimodality, marked by the blue arrow above.
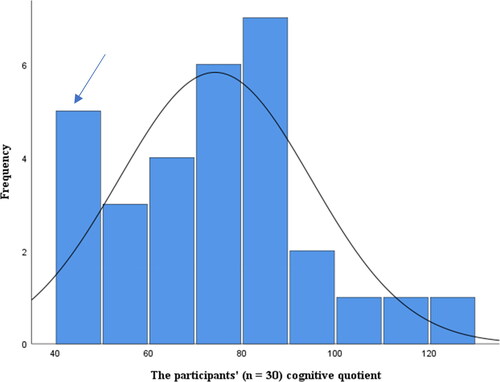
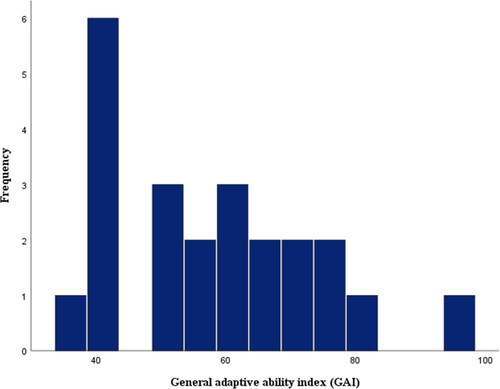
Figure 2. Distribution of the parental reports on Vineland adaptive ability Scale of the participants’ overall adaptive behavior abilities (GAI). The distribution was based on 22 reports. Twelve participants were eliminated because of their age, and 5 participants were already excluded to avoid compromising the validity of the data set. According to the tests of normality the data set appeared normally distributed.
Tests of normality indicated a bimodal distribution of the CQ scores, signifying a sample comprised of 2 groups. Analyses related to a two-group design (preschoolers and the others) complemented the results. ANOVA explored the influence of age on test performance with Bonferroni corrected post hoc test across the age groups, including preschoolers, school children (age 6.0–12.11), adolescents (ages between 13.0 and 19.11), and adults (from age 20). Regularized Exploratory Bifactor Analysis, a procedure proven appropriate for factor extraction of small samples [Citation78,Citation79] investigated any instances of similarities and discrepancies between the participants’ performances and the norm samples’ factor structure. The method is well recognized within statistical science and challenges the conventional view of sample size (i.e. typically recommended above 100) in factor analysis.
SPSS software (version 29.0) was used for the statistical computations and graphics. The chosen significance level was p < .05.
Results
Findings related to criterion A of intellectual disability
Thirty-five participants underwent cognitive assessment. However, five needed modified test conditions compromising test validity and were omitted from the numerical analyses. Based on the results from the other 30 performances (Wechsler), the statistical analyses gave a mean CQ score of 74.2 (SD = 20.5). Eleven of these scores corresponded to criterion A, which implies CQ estimates below the established cutoff of 70.
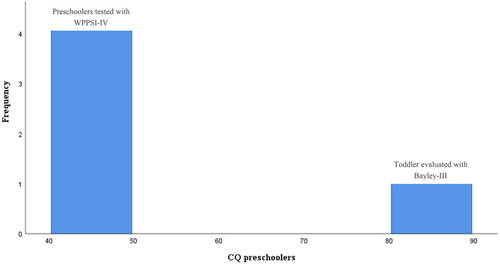
Analysis of bimodality revealed age as an underlying phenomenon and possible predictor of test performance. A two-group design revealed distribution values and graphics in favor of this assumption. Besides the one tested with Bayley-III, all the preschoolers presented CQ scores with low variance (M = 45.8, SD = 2.6) ().
Figure 3. Distribution of the preschoolers’ CQ scores after their performances on either WPPSI (n = 4) or Bayley-III (n = 1). Four preschoolers out of 5 was assessed with WPPSI-IV. One child was assessed with Bayley-III because of the young age. Bayley-III is not an intelligence scale, like the Wechsler scales, but gives information about whether a child’s developmental trajectory is proceeding as expected, relative to same-age peers. This may be part of the reason for the differences in performances, as illustrated by the two blue bars.
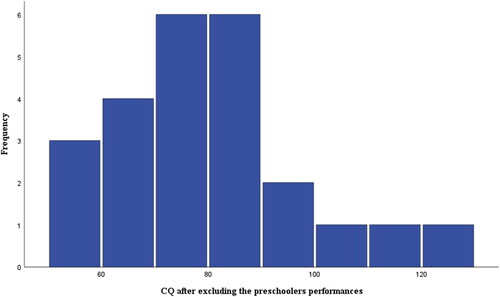
Excluding the preschoolers’ CQ estimates from the data set increased the samples’ mean significantly (M = 79.7, SD = 17.5, p < .05). The normality tests remained satisfactory (). Age-group comparisons revealed that adults obtained higher mean CQ (M = 85.2) than adolescents (M = 77.1), who, in turn, outperformed the elementary children (M = 72.4). Age-group differences overall were significant (F(3,26) = 3.8, p = .02). Post hoc testing identified only the difference between the adults’ and preschoolers’ CQ as significant (p = .02).
Figure 4. Distribution of the participants’ CQ scores after excluding the preschoolers’ performances due to the indicated bimodality. Tests of normality appeared satisfactory also after the exclusion (n = 25).
The regularized explorative bifactor analysis of the findings on WISC-V organized the test items into perceptual reasoning (factor 1) and verbal abilities (factor 2) after eliminating items demonstrating cross-loadings above .3 (). Reliability statistics proved satisfactory for both factors (α > .8), with factor 1 consisting of Blocks, Matrices, Figure weights, and Picture memory and factor 2 consisting of Receptive vocabulary, Information, and Verbal reasoning. In comparison, applying the original 5-factor structure of WISC-V gave cross-loadings above .3 between all the tests except the test items Coding and Letter-number sequence appearing as separate factors (). Using the same procedure with performances on WAIS-IV revealed a 4-factor structure corresponding more to the norm samples’ factor structure on WAIS-IV with different factors representing three domains. The only missing factor was related to memory functions ( and ).
Table 4. Explorative bifactor analysis of the participants’ performances on WISC-V.
Table 5. Factor analysis of the performances on WISC-V with 5 fixed factors.
Table 6. Explorative bifactor analysis of the performances on WAIS-IV.
Table 7. Factor analysis of the performances on WAIS-IV with 4 fixed factors.
Findings related to criterion B of intellectual disability
The upper age range of the Norwegian version of Vineland is 21.11, eliminating 12 participants (outside the age range). Because of validity concerns, four had already been excluded from the data set. The last of these five had a GAI estimate but lacked an estimate of CQ and kept from further analyses. Analyses of the remaining 22 parental Vineland reports gave a mean GAI estimate of 57.6 (SD = 15.6), which proved significantly lower than CQ (r = .59, p = .005). Among the domain-related indices, only the communication index demonstrated significant relation to CQ (r = .5, p < .05). Nineteen of the GAI scores appeared below 70, corresponding to criterion B’s cutoff.
No significant effect emerged by excluding the preschoolers from the others (GAI of 60.9, SD = 15.2), and the kurtosis estimate (above 1) and the distribution curve appeared less satisfactory. Age was only a potential predictor of GAI when comparing the adolescents’ scores (M = 63.4) with those of the school children (M = 51.8). For the other age groups, GAI was relatively similar.
Discussion
Findings revealed that about half of the participants in this study would potentially have obtained a diagnosis of intellectual disability between a moderate and a profound degree with norm-referenced assessments. The percentage would correspond with often recited estimates in CHARGE literature [Citation10]. However, the hypothesis of this study assumed an increased risk of false positives included in this prevalence because of the incongruence between the CHARGE characteristics and the neurotypical diagnostic premises. Findings related to each criterion (A, B, and C) supported such an assumption [Citation2].
First, the applicability of an assessment and diagnosis of intellectual disability depends on whether the obtained results reflect actual cognitive deficits marginally influenced by other factors or processes. When the individual or population in question deviates markedly from the normative sample, such as in this study, the representativeness and construct validity can be compromised. The participants’ normally distributed scores confirmed them as representative of individuals with CHARGE. However, the interpretability of scores also depends on whether there is a bidirectional representativeness between them and the norm sample of the chosen measure. With graphics displaying a CQ curve shifting horizontally to the left, and descriptive statistics presenting a group mean of 74.2 with a standard deviation of 20.5, the participants’ performances turned out significantly different than the Wechsler scales’ numeric (M = 100, SD = 15) [Citation5–7].
Further investigation of the participants’ scores revealed significant relations across indices and subtests without any sign of clustering. In addition, few significant differences appeared between the indices. Even though the sample in the present study was too small for conventional factor analysis, the results from regularized exploratory procedure WISC-V indicated a 2-factor solution rather than the original 5-factor structure. Meanwhile, the performance on WAIS-IV revealed a 4-factor structure more in accordance with the adult Wechsler tests’ original structure. For the clinician, this leads to uncertainties about the underlying processes of each score, making interpretation challenging. Because of the positive effect of age on the performance level, the adults’ CQ deviated less from those of the norm sample, implying higher interpretability and applicability of the adults’ scores compared to the younger age groups.
The second support for the hypothesis involved the validity of observation-based evaluations of adaptive behavior for individuals with atypical development. In this study, parents’ judgment on Vineland (GAI) was significantly lower than the participants’ test performances (CQ), a notable finding since the relative strengths of these estimates often are reversed in other congenital conditions [Citation80]. However, this is an expected finding due to the consequences of the sensorimotor challenges in CHARGE [Citation22,Citation81]. In typical developed children and adolescents, a positive association between psychomotoric function and brain maturation is assumed [Citation82]. The relation is a premise underlying pediatric clinical assessments and observational-based measures like Vineland. For individuals with CHARGE, these associations are less straightforward. Their unique genotype induces changes within structures and functions, influencing their environment and experiential learning [Citation83]. Exposure to different developmental conditions impacts neurobiological processes influencing brain development [Citation84,Citation85]. Over time a looping effect occurs, making predictions of outcomes challenging. For example, a significantly delayed onset of independent walking [Citation62], communicative challenges [Citation29], and language delay [Citation86] directly affect the experiential learning of children with CHARGE [Citation58]. In the present study, participants demonstrated significantly delayed gross motor functions and communication and language skills (above age 3). While compensatory abilities may be advanced in children with CHARGE, setbacks and gaps are often demonstrated within more common adaptive behaviors [Citation87]. Unfortunately, the latter is the target for evaluation in most observation-based measures. This incongruence can lead to an unjust lowering of the performance-based results and increase the risk of diagnostic fallacies. Consequently, global evaluations of adaptive behavior for individuals with CHARGE as a diagnostic parameter may, at worst, lead to false positives.
The third supportive finding of this study hypothesis involves diagnostic timing. The differences between the participants’ CQ scores and the norm sample decreased with age. While the adults obtained the highest results, the preschoolers’ performances were associated with evenly low scores (i.e. a floor effect). Despite being age-relevant, the instrument could not capture the variance in the children’s competency (WPPSI-IV). These floor effects also illustrate a large gap between actual competancy and the expected performance level (the norms). The discrepancy seems most prominent in early childhood, decreasing to adulthood, and may reflect an overall effect of atypical features on development.
Limitations
The present study had several limitations. The primary assessment accommodations involved test administration in sign language. Paraphrasing instructions and questions became necessary due to the many linguistic differences between Norwegian sign language and Norwegian speech. Despite the presence of sign language teachers or assistants during testing to validate the communication, validity issues may have affected the results.
Because of the need to substitute the original norms with Scandinavian, the third version of Vineland was unavailable in Norway at the time of the assessment. Using VABS-III may have added or changed some of the results.
Different living arrangements sometimes made it necessary to conduct semi-structured interviews with caregivers other than the parents, which can have influenced their reports and the global estimate.
Conclusions and implications
Findings from this study supported the assumption that individuals with CHARGE are at increased risk of underestimation of intellectual ability due to their atypical characteristics not accounted for in norm-referenced assessments.
Age was identified as a predictor of performance level and was an influential factor in the manifestations of atypicality. The effect of the atypical features seemed most prominent in the youngest children, particularly the preschoolers, subsiding towards adulthood.
The main implication of the present study relates to the timing of the final assessment and diagnosis of intellectual disability in individuals with CHARGE, which should wait until the individual reaches cognitive maturity, generally not until young adulthood, to ensure validity. As a part of this final diagnostic evaluation and to optimize developmental conditions, school and/or school personnel should monitor cognitive progress by systematic cognitive evaluations from early childhood. In addition to providing educational guidance, such longitudinal follow-up enables clinicians to evaluate the occurrence of cognitive maturity demonstrated by stabilization of functions over time.
Employing the upper confidence interval during norm-referenced assessments can account for some of the negative consequences of sub-optimal assessment conditions and potential measurement errors. While performance-based assessment with appropriate timing can increase diagnostic validity, pure observational-based overall evaluations of the adaptive behavior of individuals with CHARGE seem questionable as a diagnostic parameter.
In diagnostic evaluations, complementary performance-based testing should precede measures based on clinical observations. The choice of instruments depends on the individual’s cognitive profile on earlier measures. Any results at the lowest bound of the test’s distribution area (i.e. floor effects) deserve special attention and further investigation, as these estimates may camouflage specific cognitive challenges and unjustly reduce the individual’s global cognition scores. In the case of floor effects within the verbal domain, complementary testing of abilities related to verbal function are warranted, such as verbal comprehension, verbal memory, and problem-solving.
Considering that atypical and delayed progress are present in many congenital disorders besides CHARGE, this study’s validity issues and suggested implications may also apply across conditions. These are important topics for future research efforts.
Ethics statement
This study was approved by the Norwegian Regional Committees for Medical and Health Research Ethics (#2020/43412) and conforms to the recognized standards of the Declaration of Helsinki.
Acknowledgements
We would like to thank all the participants and their significant others for taking part in this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Farmer CA, Thurm A, Troy JD, et al. Comparing ability and norm-referenced scores as clinical trial outcomes for neurodevelopmental disabilities: a simulation study. J Neurodev Disord. 2023;15(1):4. Article 4. doi: 10.1186/s11689-022-09474-6.
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. (5th ed.). Arlington (VA): American Psychiatric Association; 2013.
- Bayley N. Bayley scales of infant and toddler development. (3rd ed.). Sweden: Pearson Assessment; 2005.
- Sparrow SS, Cicchetti DV, Balla DA. Vineland adaptive behavior scales: Norwegian version. (2nd ed.). Stockholm: NCS Pearson; 2011.
- Wechsler D. Wechsler adult intelligence scale: Norwegian version. (4th ed.). Stockholm: Pearson Assessment; 2008.
- Wechsler D. (WPPSI-IV): Wechsler preschool and primary scale of intelligence: Norwegian version. (4th ed.). Stockholm: Pearson Assessment; 2012.
- Wechsler D. (WPPSI-IV): Wechsler intelligence scale for children: Norwegian version. (5th ed.). Stockholm: Pearson Assessment; 2014.
- Karmiloff-Smith A. Nativism versus neuro constructivism: rethinking the study of developmental disorders. Dev Psychol. 2009;45(1):56–63. doi: 10.1037/a0014506.
- D’Souza D, Karmiloff-Smith A. When modularization fails to occur: a developmental perspective. Cogn Neuropsychol. 2011;28(3–4):276–287. doi: 10.1080/02643294.2011.614939.
- Thomas AT, Waite J, Williams CA, et al. Phenotypic characteristics and variability in CHARGE syndrome: a PRISMA compliant systematic review and meta-analysis. J Neurodev Disord. 2022;14(1):49. doi: 10.1186/s11689-022-09459-5.
- Hittner HM, Hirsch NJ, Kreh GM, et al. Colobomatous microphthalmia, heart disease, hearing loss, and mental retardation-a syndrome. J Pediatr Ophthalmol Strabismus. 1979;16(2):122–128. doi: 10.3928/0191-3913-19790301-10.
- Davenport SL, Hefner MA, Mitchell JA. The spectrum of clinical features in CHARGE syndrome. Clin Genet. 1986;29(4):298–310. doi: 10.1111/j.1399-0004.1986.tb01258.x.
- Oley CA, Baraitser M, Grant DB. A reappraisal of the CHARGE association. Journal of Medical Genetics. 1988;25(3):147–156. (1)., (), –. doi: 10.1136/jmg.25.3.147.
- Asher BF, McGill TJ, Kaplan L, et al. Airway complications in CHARGE association. Archives of Otolaryngology–Head & Neck Surgery. 1990;116(1):15.
- Blake KD, Russell-Eggitt IM, Morgan DW, et al. Who’s in CHARGE? Multidisciplinary management of patients with CHARGE association. Arch Dis Child. 1990;65(2):217–223. doi: 10.1136/adc.65.2.217.
- Harvey AS, Leaper PM, Bankier A. CHARGE association: clinical manifestations and developmental outcome. Am J Med Genet. 1991;39(1):48–55. doi: 10.1002/ajmg.1320390112.
- Blake KD, Brown D. CHARGE association looking at the future, the voice of a family support group. Child Care Health Dev. 1993;19(6):395–409. doi: 10.1111/j.1365-2214.1993.tb00744.x.
- Tellier AL, Cormier-Daire V, Abadie V, et al. CHARGE syndrome: report of 47 cases and review. Am J Med Genet. 1998;76(5):402–409. doi: 10.1002/(SICI)1096-8628(19980413)76:5<402::AID-AJMG7>3.0.CO;2-O.
- Roger G, Morisseau-Durand MP, Van Den Abbeele T, et al. The CHARGE association: the role of tracheotomy. Arch Otolaryngol Head Neck Surg. 1999;125(1):33–38. doi: 10.1001/archotol.125.1.33.
- Raqbi F, Le Bihan C, Morisseau-Durand MP, et al. Early prognostic factors for intellectual outcome in CHARGE syndrome. Dev Med Child Neurol. 2003;45(7):483–488. doi: 10.1111/j.1469-8749.2003.tb00944.x.
- Hartshorne TS, Cypher AD. Challenging behavior in CHARGE syndrome. MHDD. 2004;7(2):41–52.
- Salem-Hartshorne N, Jacob S. Characteristics and development of children with CHARGE association/syndrome. J Early Interv. 2004;26(4):292–301. doi: 10.1177/105381510402600405.
- Bernstein V, Denno LS. Repetitive behaviors in CHARGE syndrome: differential diagnosis and treatment options. Am J Med Genet A. 2005;133A(3):232–239. doi: 10.1002/ajmg.a.30542.
- Blake KD, Salem-Hartshorne N, Daoud MA, et al. Adolescent and adult issues in CHARGE syndrome. Clin Pediatr. 2005;44(2):151–159. doi: 10.1177/000992280504400207.
- Issekutz KA, Graham JM, Prasad C, et al. An epidemiological analysis of CHARGE syndrome: preliminary results from a Canadian study. Am J Med Genet. 2005;133A(3):309–317. doi: 10.1002/ajmg.a.30560.
- Smith IM, Nichols SL, Issekutz K, et al. Behavioral profiles and symptoms of autism in CHARGE syndrome: preliminary Canadian epidemiological data. Am J Med Genet A. 2005;133A(3):248–256. doi: 10.1002/ajmg.a.30544.
- Souriau J, Gimenes M, Blouin C, et al. CHARGE syndrome: developmental and behavioral data. Am J Med Genet A. 2005;133A(3):278–281. doi: 10.1002/ajmg.a.30549.
- Strömland K, Sjögreen L, Johansson M, et al. CHARGE association in Sweden: malformations and functional deficits. Am J Med Genet A. 2005;133A(3):331–339. doi: 10.1002/ajmg.a.30563.
- Thelin JW, Fussner JC. Factors related to the development of communication in CHARGE syndrome. Am J Med Genet A. 2005;133A(3):282–290. doi: 10.1002/ajmg.a.30550.
- Johansson M, Råstam M, Billstedt E, et al. Autism spectrum disorders and underlying brain pathology in CHARGE association. Dev Med Child Neurol. 2006;48(1):40–50. doi: 10.1017/S0012162206000090.
- Jongmans MCJ, Admiraal RJ, Van Der Donk KP, et al. CHARGE syndrome: the phenotypic spectrum of mutations in the CHD7 gene. J Med Genet. 2006;43(4):306–314. doi: 10.1136/jmg.2005.036061.
- Dobbelsteyn C, Peacocke SD, Blake K, et al. Feeding difficulties in children with CHARGE syndrome: prevalence, risk factors, and prognosis. Dysphagia. 2008;23(2):127–135. doi: 10.1007/s00455-007-9111-6.
- Reda NM, Hartshorne TS. Attachment, bonding, and parental stress in CHARGE syndrome. MHDD. 2008;11(2008):1.
- Wincent J, Holmberg E, Strömland K, et al. CHD7 mutation spectrum in 28 swedish patients diagnosed with CHARGE syndrome. Clin Genet. 2008;74(1):31–38. doi: 10.1111/j.1399-0004.2008.01014.x.
- Hartshorne TS, Heussler HS, Dailor AN, et al. Sleep disturbances in CHARGE syndrome: types and relationships with behavior and caregiver well-being. Dev Med Child Neurol. 2009;51(2):143–150. doi: 10.1111/j.1469-8749.2008.03146.x.
- Wulffaert J, Scholte EM, Dijkxhoorn YM, et al. Parenting stress in CHARGE syndrome and the relationship with child characteristics. J Dev Phys Disabil. 2009;21(4):301–313. doi: 10.1007/s10882-009-9143-y.
- Wessels K, Bohnhorst B, Luhmer I, et al. Novel CHD7 mutations contributing to the mutation spectrum in patients with CHARGE syndrome. Eur J Med Genet. 2010;53(5):280–285. doi: 10.1016/j.ejmg.2010.07.002.
- Dammeyer J. Development and characteristics of children with usher syndrome and CHARGE syndrome. Int J Pediatr Otorhinolaryngol. 2012;76(9):1292–1296. doi: 10.1016/j.ijporl.2012.05.021.
- Deuce G, Howard S, Rose S, et al. A study of CHARGE syndrome in the UK. Br J Vis Impair. 2012;30(2):91–100. doi: 10.1177/0264619612443883.
- Hartshorne N, Hudson A, MacCuspie J, et al. Quality of life in adolescents and adults with CHARGE syndrome. Am J Med Genet A. 2016;170(8):2012–2021. doi: 10.1002/ajmg.a.37769.
- Husu E, Hove HD, Farholt S, et al. Phenotype in 18 Danish subjects with genetically verified CHARGE syndrome. Clin Genet. 2013;83(2):125–134. doi: 10.1111/j.1399-0004.2012.01884.x.
- Lasserre E, Vaivre-Douret L, Abadie V. Psychomotor and cognitive impairments of children with CHARGE syndrome: common and variable features. Child Neuropsychol. 2013;19(5):449–465. doi: 10.1080/09297049.2012.690372.
- Hsu P, Ma A, Wilson M, et al. CHARGE syndrome: a review. J Paediatr Child Health. 2014;50(7):504–511. doi: 10.1111/jpc.12497.
- Shoji Y, Ida S, Etani Y, et al. Endocrinological characteristics of 25 Japanese patients with CHARGE syndrome. Clin Pediatr Endocrinol. 2014;23(2):45–51. doi: 10.1297/cpe.23.45.
- Sohn YB, Ko JM, Shin CH, et al. Cerebellar vermis hypoplasia in CHARGE syndrome: clinical and molecular characterization of 18 unrelated Korean patients. J Hum Genet. 2016;61(3):235–239. doi: 10.1038/jhg.2015.135.
- Hale CL, Niederriter AN, Green GE, et al. A typical phenotypes associated with pathogenic CHD7 variants and a proposal for broadening CHARGE syndrome clinical diagnostic criteria. Am J Med Genet A. 2016;170A(2):344–354. doi: 10.1002/ajmg.a.37435.
- Deuce G. The education of learners with CHARGE syndrome. British Journal of Special Education. 2017;44(4):376–393. doi: 10.1111/1467-8578.12183.
- Legendre M, Abadie V, Attié-Bitach T, et al. Phenotype and genotype analysis of a French cohort of 119 patients with CHARGE syndrome. Am J Med Genet C Semin Med Genet. 2017;175(4):417–430. doi: 10.1002/ajmg.c.31591.
- Shiohama T, McDavid J, Levman J, et al. Quantitative brain morphological analysis in CHARGE syndrome. Neuroimage Clin. 2019;23(2019):101866. Article 101866. doi: 10.1016/j.nicl.2019.101866.
- Abadie V, Hamiaux P, Ragot S, et al. Should autism spectrum disorder be considered part of CHARGE syndrome? A cross-sectional study of 46 patients. Orphanet J Rare Dis. 2020;15(1):136–136. doi: 10.1186/s13023-020-01421-9.
- Cheng SS, Luk HM, Chan DK, et al. CHARGE syndrome in nine patients from China. Am J Med Genet A. 2020;182(1):15–19. doi: 10.1002/ajmg.a.61398.
- Blake KD, Prasad C. CHARGE syndrome. Orphanet J Rare Dis. 2006;1(1):34–34. doi: 10.1186/1750-1172-1-34.
- Hodorovich DR, Lindsley PM, Berry AA, et al. Morphological and sensorimotor phenotypes in a zebrafish CHARGE syndrome model are domain-dependent. Genes Brain Behav. 2023;22(3):e12839. doi: 10.1111/gbb.12839.
- Filippi R, Karmiloff-Smith A. What can neurodevelopmental disorders teach us about typical development. In: Marshall CR, editor. Current issues in developmental disorders. London: Psychology Press; 2013. p. 193–209.
- Vissers LE, van Ravenswaaij CM, Admiraal R, et al. Mutations in a new member of the chromodomain gene family cause CHARGE syndrome. Nat Genet. 2004;36(9):955–957. doi: 10.1038/ng1407.
- da Costa Monsanto R, Knoll RM, de Oliveira Penido N, et al. Otopathologic abnormalities in CHARGE syndrome. Otolaryngol Head Neck Surg. 2023;166(2):363–372. doi: 10.1177/01945998211008911.
- Onesimo R, Ricci D, Agazzi C, et al. Visual function and ophthalmological findings in CHARGE syndrome: revision of literature, definition of a new clinical spectrum and genotype phenotype correlation. Genes. 2021;12(7):972. doi: 10.3390/genes12070972.
- Imel G, Hartshorne TS, Slavin LJ, et al. Participation in and barriers to recreation participation in CHARGE syndrome. Palaestra. 2020;34(1):38–43.
- The Nordic Deafblind Cooperation Committee. Nordic definition of deafblindness. 2016. Available from: http://www.fsdb.org/Filer/DBNSK%20English.pdf.
- Gottlieb G. 1992. Individual development and evolution: the genesis of novel behavior. New York: Oxford University Press.
- Haibach PS, Lieberman LJ. Balance and self-efficacy of balance in children with CHARGE syndrome. J Vis Impair Blind. 2013;107(4):297–309. doi: 10.1177/0145482X1310700406.
- Perreault M, Haibach-Beach P, Lieberman L, et al. Motor competence in children with CHARGE syndrome. Res Pract Persons Severe Disabil. 2021;46(2):67–76. doi: 10.1177/1540796921998011.
- Hallemans A, Ortibus E, Truijen S, et al. Development of independent locomotion in children with a severe visual impairment. Res Dev Disabil. 2011;32(6):2069–2074. doi: 10.1016/j.ridd.2011.08.017.
- Klingenberg C. Medical aspects of CHARGE syndrome. Siggerud, Norway: Frambu Resource Centre for Rare Disorders; 2017.
- Olusanya BO, Davis AC, Hoffman HJ. Hearing loss grades and the international classification of functioning, disability and health. Bull World Health Organ. 2019;97(10):725–728. doi: 10.2471/BLT.19.230367.
- Hatt SR, Leske DA, Castañeda YS, et al. Development of pediatric eye questionnaires for children with eye conditions. Am J Ophthalmol. 2019;200(2019):201–217. doi: 10.1016/j.ajo.2019.01.001.
- von Tetzchner, S., Martinsen, H, editors. Introduction to augmentative and alternative communication: sign teaching and the use of communication aids for children, adolescents and adults with developmental disorders. (2nd ed.). London: Whurr Publisher; 2000.
- Sireci S, O’Riordan M. Comparability when assessing individuals with disabilities. In: Berman AI, Haertel EH, Pellegrino JW, editors. Comparability of large-scale educational assessments. Issues and recommendations. Washington DC: National Academy of Education; 2020. p. 177–204.
- American Educational Research Association. Standards for educational and psychological measurement. Washington (DC): American Psychological Association & National Council on Measurement in Education; 2014.
- DePascale C, Gong B. Comparability of individual student scores on the “same test”. In: Berman AI, Haertel EH, Pellegrino JW, editors. Comparability of large-scale educational assessments. Issues and recommendations. Washington DC: National Academy of Education; 2020. p. 25–47.
- Elliott SN, Kettler RJ. Item and test design considerations for students with special needs. In: Lane S, Haladyna T, Raymond M, editors. Handbook of test development. Washington (DC): National Council on Measurement in Education; 2016. p. 374–391.
- Fuchs LS, Fuchs D, Eaton SB, et al. Supplementing teacher judgments about test accommodations with objective data sources. School Psych Rev. 2000;29(1):65–85. doi: 10.1080/02796015.2000.12085998.
- Chatzidamianos G, Burns D, Andriopoulou P, et al. The challenges and facilitators to successful translation and adaptation of written self-report psychological measures into sign languages: a systematic review. Psychol Assess. 2021;33(11):1100–1124. doi: 10.1037/pas0001061.
- Marschark M, Knoors H. Educating deaf children: language, cognition, and learning. Deaf Educ Int. 2012;14(3):136–160. doi: 10.1179/1557069X12Y.0000000010.
- Marschark, M., & Knoors, H. (Eds.) 2020. The oxford handbook of deaf studies in learning and cognition. Oxford: Oxford University Press.
- Cronbach LJ, Meehl PE. Construct validity in psychological tests. Psychol Bull. 1955;52(4):281–302. doi: 10.1037/h0040957.
- Kaat AJ, Bishop S, Condy E, et al. Prerequisite skills in cognitive testing: innovations in theory and recommendations for practice. Cogn Dev. 2021;58(2021):101038. doi: 10.1016/j.cogdev.2021.101038.
- Giordano C, Ones DS, Waller NG, et al. Exploratory bifactor measurement models in vocational behavior research. J Vocat Behav. 2020;120:103430. doi: 10.1016/j.jvb.2020.103430.
- Jung S, Seo DG, Park J. Regularized exploratory bifactor analysis with small sample sizes. Front Psychol. 2020;11(2020):507. doi: 10.3389/fpsyg.2020.00507.
- Tassé MJ, Luckasson R, Schalock RL. The relation between intellectual functioning and adaptive behavior in the diagnosis of intellectual disability. Intellect Dev Disabil. 2016;54(6):381–390. doi: 10.1352/1934-9556-54.6.381.
- Salem-Hartshorne N, Jacob S. Adaptive behavior in children with CHARGE syndrome. Am J Med Genet A. 2005;133A(3):262–267. doi: 10.1002/ajmg.a.30546.
- Levy Y. Developmental delay reconsidered: the critical role of age-dependent, co-variant development. Front Psychol. 2018;9(2018):503. doi: 10.3389/fpsyg.2018.00503.
- Karmiloff-Smith A, D’Souza D, Dekker TM, et al. Genetic and environmental vulnerabilities in children with neurodevelopmental disorders. Proc Natl Acad Sci U S A. 2012;109(Suppl 2):17261–17265. doi: 10.1073/pnas.1121087109.
- Weinstein M, Ben-Sira L, Levy Y, et al. Abnormal white matter integrity in young children with autism. Hum Brain Mapp. 2011;32(4):534–543. doi: 10.1002/hbm.21042.
- Wolff JJ, Gu H, Gerig G, et al. Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. Am J Psychiatry. 2012;169(6):589–600. doi: 10.1176/appi.ajp.2011.11091447.
- Vesseur A, Langereis M, Free R, et al. Influence of hearing loss and cognitive abilities on language development in CHARGE syndrome. Am J Med Genet A. 2016;170(8):2022–2030. doi: 10.1002/ajmg.a.37692.
- Salem-Hartshorne N. 2003. Developmental delay in CHARGE syndrome: a four-year follow-up (publication no. 3104072) [Doctor dissertation]. Michigan: Central Michigan University, ProQuest Dissertations Publishing.

