Abstract
Background
Hyperbaric oxygenation (HBO) therapy can improve locomotor dysfunction following spinal cord injury (SCI). Emerging evidence has demonstrated that sirtuin1 (SIRT1) exerts protective effects on neurons. However, whether HBO alleviates locomotor dysfunction by regulating SIRT1 is unclear.
Methods
The traumatic SCI animal model was performed on the adult Sprague-Dawley rats. The Basso, Beattie Bresnahan (BBB) locomotor rating scale was used to evaluate the open-field locomotor function. Western blot, real-time quantitative reverse transcription polymerase chain reaction, SIRT1 activity assay, and enzyme-linked immunosorbent assays were performed to explore the molecular mechanisms.
Results
We found that series HBO therapy significantly improved locomotor dysfunction and ameliorated the decreased mRNA, protein, and activity of spinal cord SIRT1 induced by traumatic SCI injury in rats. In addition, intraperitoneal injection of SIRT1 inhibitor EX-527 abolished the beneficial effects of series HBO treatment on locomotor deficits. Importantly, series HBO treatment following the traumatic SCI injury inhibited the inflammatory cascade and apoptosis-related protein, which was retained by EX-527 and enhanced by SRT1720. Furthermore, EX-527 blocked the enhanced induction of autophagy series with the HBO application.
Conclusion
These findings demonstrated a new mechanism for series HBO therapy involving activation of SIRT1 and subsequent modulation of the inflammatory cascade, apoptosis, and autophagy, which contributed to the recovery of motor dysfunction.
Introduction
Traumatic spinal cord injury (SCI) is a catastrophic and disabling disease that is primarily caused by external physical impacts such as traffic accidents, sports injuries, falls, and work accidents (referred to as primary injury) [Citation1]. The primary injury is usually temporary and irreversible [Citation2], however, it leads to a secondary injury, which results in permanent neurological deficits including motor and sensory dysfunction [Citation1, Citation3]. The direct damage to spinal cord cells initiates the complicated and devastating secondary injury cascade which may contribute to permanent function impairment [Citation4], which can lead to permanent functional impairment and psychological debilitation [Citation5]. During this cascade, the death of neurons and glia, as well as inflammation caused by the primary damage, occur cyclically [Citation4]. Importantly, uncontrolled posttraumatic inflammation and apoptosis of neurons further worsen motor dysfunction [Citation6]. Therefore, controlling the posttraumatic inflammation cascade and apoptosis-related proteins can greatly benefit the recovery of motor dysfunction.
Hyperbaric oxygenation (HBO) involves administering 100% oxygen at a pressure between one and three times that of atmospheric pressure. HBO therapy has been used both clinically and experimentally to enhance neurological recovery in brain injuries [Citation7, Citation8] and cerebral ischemia [Citation9, Citation10]. In recent years, HBO therapy has been proposed as a potential treatment for spinal cord injuries (SCI) due to its neuroprotective effects on neurons [Citation11]. For instance, HBO therapy has been shown to improve locomotor recovery by regulating the levels of monocyte chemoattractant protein-1 (MCP-1, also known as CCL2), a crucial inflammatory cytokine to recruit inflammatory cells (neutrophil and macrophage, etc.) [Citation12]. Additionally, HBO therapy has been found to significantly reduce neuronal apoptosis in injured spinal cord tissue [Citation13]. Additionally, HBO therapy has been found to significantly reduce neuronal apoptosis in injured spinal cord tissue [Citation14–16], but the mechanism of the effect of HBO therapy on traumatic spinal cord injury is still largely unknown.
Sirtuin1 (SIRT1) protein is a highly conserved nicotinamide adenine dinucleotide (NAD+)-dependent deacetylase found in bacteria and humans [Citation17]. SIRT1 is known to modulate the deacetylation of histones [Citation18] and other substrates, such as p53 [Citation19], nuclear factor kappa B (NF-κB) [Citation20], peroxisome proliferator-activated receptor γ (PPARγ) [Citation20] and others. Through the regulation of these proteins, SIRT1 is involved in various cellular processes, such as energy metabolism, cell survival, autophagy, inflammation, and apoptosis [Citation20, Citation21]. Emerging evidence demonstrated that SIRT1 plays a protective role in Alzheimer’s [Citation22] and Parkinson’s [Citation23], potentially due to its function in anti-inflammation [Citation19], anti-apoptosis [Citation24], and genomic stability [Citation17]. However, it is currently unknown whether HBO therapy can improve locomotor dysfunction caused by traumatic SCI through the regulation of SIRT1. Therefore, in this study, we aim to investigate the hypothesis that HBO modulates SIRT1 and subsequently ameliorates motor dysfunction induced by traumatic SCI.
Materials and methods
Animals
Adult female Sprague-Dawley rats, weighing 260 to 320 g, were obtained from The Medical Experimental Animal Center of Guangdong Province (Guangzhou, China). The animals were housed under a 12-h light/dark cycle with free access to food and water at a constant room temperature of 26 °C ± 1 °C. The rats were randomly separated into different groups. All experimental protocols were approved and conducted according to the guidelines of the General Hospital of Southern Theater Command Animal Ethic Committee by the National Institutes of Health on animal care and ethical guidelines.
Surgical and HBO treatment
The traumatic SCI model was established according to a previous study [Citation15] with minor changes. Briefly, the surgical site was shaved and swabbed with an iodine solution and then with 75% alcohol after animals were anesthetized intraperitoneally with 50 mg/kg sodium pentobarbital. The laminectomy was conducted to expose the T9–11 spinal cord without rupturing the dura mater after a midline skin incision was made to expose the T9–11 spinal column. Then, traumatic spinal cord injury was induced with an impacting stick (10 g) from a height of 10 mm (10-g/cm force) onto the exposed spinal cord. After the incision was sutured, 5 mL of saline was immediately administered via intraperitoneal injection to replace the blood loss. In addition, a heater was used to monitor and maintain the body temperature of the rats at 37 °C for 24h to reduce postoperative mortality. The rats were assisted in urinating twice daily until the micturition reflex had recovered. For HBO treatment, the chamber was flushed with pure oxygen for 10 min, and then the pressure was increased at a rate of 0.2 MPa (2 ATA) per 20 min. The oxygen concentration in the chamber was maintained at 90%. After continuous oxygen treatment for 60 min, decompression was performed at a uniform rate within 20 min. HBO therapy was conducted once daily for 10 consecutive days. The time point of administration of series HBO treatment for rats is at posttraumatic 8h.
Drugs administration
The SIRT1 activator SRT1720 and inhibitor EX-527 were purchased from Selleck Chemical (Houston, TX) and dissolved in 1% dimethyl sulfoxide (DMSO). All of the drugs were diluted with 0.9% saline to the appropriate concentration. After induced traumatic SCI, SRT1720 and EX-527 were intraperitoneally injected (i.p.) at a concentration of 150 mg/kg and 100 mg/kg respectively once daily for 7 days.
Behavior test
The Basso, Beattie Bresnahan (BBB) locomotor rating scale was used to evaluate the open-field locomotor function as previously described [Citation15]. In brief, rats were exposed to the behavioral testing facility daily for 1 week to acclimate them to open-field exploration before the SCI surgery was performed. Then the BBB scores were assessed according to the BBB locomotor rating scale. The examiners were blinded to the type of treatment each animal received.
Tissue collection
After animals were anesthetized with 5% pentobarbital sodium (50 mg/kg), the spinal cord (T9-T11) tissues were removed and frozen in liquid nitrogen for the western blot and RT-PCR analysis.
Quantitative RT-PCR
Total RNA extractions and frozen tissue specimens were conducted with TRIzol® reagent (Invitrogen). The Takara Reverse Transcription System Kit (Takara Biotechnology Co. Ltd, Japan) was used to synthesize cDNA. The quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR) was performed using the SYBR green premix kit (BioRad, Hercules, CA, USA). GAPDH was used as an internal control. The primer sequences were as follows. SIRT1 Forward: ACCTCCTCATTGTTATTGGGTCTTC, Reverse: GGCATACTCGCCACCTAACC.
Western blot
Western blot was performed as in the previous study. Generally speaking, spinal cord tissues were homogenized in 15 mmol/l Tris containing a cocktail of proteinase inhibitors and phosphatase inhibitors (Roche, USA). The extracted protein after boiled for 6 mins was separated by 10% SDS-PAGE (BioRad, Germany) and then transferred onto a PVDF membrane (Millipore, USA). Then the PVDF membrane is blocked with 5% fat-free milk for 60 min. Primary antibodies were incubated at 4 °C overnight. On the following day, the TBST is used to wash out the primary antibodies, and the membranes are incubated with secondary antibodies. The primary antibodies used in the experiment were as follows: rabbit SIRT1 (1:1000, Cell Signaling Technology#2496), rabbit NF-κB (1:2000, Abcam#ab16502), rabbit NF-κB (Acetyl K310)(1:2000, Abcam#ab218533), mouse p62 (1:1000, Cell Signaling Technology#88588), rabbit anti-ATG-5 (1:1000, Cell Signaling Technology#12994), rabbit anti-Beclin-1 (1:1500, Abcam#ab207612), rabbit anti-LC3B (1:1000, Abcam#ab48394), mouse anti-GAPDH (1:1500, Abcam#ab8245)
Enzyme-linked immunosorbent (ELISA) assay
The tissue sample was collected and immediately homogenized in an extraction buffer with a protease inhibitor. The inflammatory cytokines (TNF-α, IL-1β, IL-6, and IL-10) and caspase (caspase1, caspase3, caspase 8 and caspase 12) were detected as manufactures instruction.
SIRT1 activity assay
SIRT1 enzymatic activity was measured using a fluorescent assay kit (Cayman) according to the manufacturer’s instructions. Briefly, tissue samples were sonicated in RIPA buffer (0.7% Na deoxycholate, 0.5 M LiCl, 50 mM HEPES-KOH, pH 7.6, 1% NP-40, 1 mM EDTA) and lysates were mixed with assay buffer and substrate and incubated for 45 min at room temperature with shaking. Then, the developing solution was added and incubated for 30 min. Fluorescence was measured with a microplate reader (excitation:360 nm, emission: 465 nm; Safire 2, Tecan).
Data and analysis
Data were presented as the mean values ± SEM and analyzed with GraphPad Prism™, version 6.00 software (GraphPad, La Jolla, CA, USA). The Student’s t-test or one-way ANOVA was used to determine the statistical significance of differences between two groups or among variant groups, respectively. A p-value < 0.05 was considered statistically significant.
Results
The reduced expression and activity of SIRT1 induced by traumatic SCI were significantly inhibited following the HBO treatment
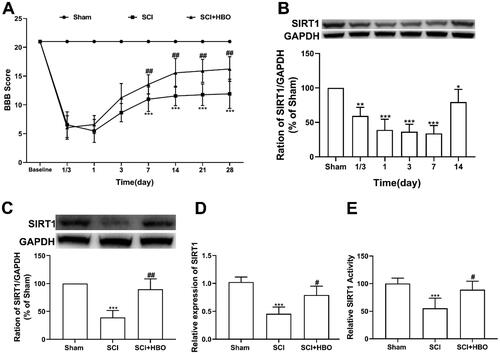
Consistent with a previous study [Citation15], we found that the application of series HBO treatment began at 8 h following the SCI injury in rats and lasted for 7 continuous days which significantly alleviated the SCI-induced locomotor dysfunction (). As shown in , a decreased protein level of spinal cord SIRT1 was induced by SCI injury in rats since the 8h (1/3 day) which lasted at least at day 14 postinjury. To further evaluate the role of SIRT1 in the effects of HBO on SCI-induced locomotor dysfunction, we observed that the reduced expression of mRNA and protein of spinal cord SIRT1 in SCI rats on day 7 postinjury was dramatically upregulated by HBO treatment (). In addition, the SIRT1 activity assay revealed that series HBO administration overturned the decrease of SIRT1 activity on day 7 postinjury ().
Figure 1. HBO therapy enhanced the decreased expression and activity of spinal cord SIRT1 induced by traumatic SCI. (A) The locomotor dysfunction was significantly improved after traumatic SCI rats were applied with series HBO therapy (n = 12/per group). (B) The protein level of spinal cord SIRT1 began to reduce at 8 h (1/3 day) and lasted at least at day 14 after the SCI injury established (n = 4/per group). (C–E) HBO treatment for consecutive 7 days significantly enhanced the decreased protein, mRNA and activity of SIRT1 in spinal cord (n = 5/per group). *p<0.05, **p<0.01, ***p<0.001 compared with sham group, #p<0.05, ##p<0.01 compared with SCI group.
SIRT1 was implicated in the benefit of series HBO treatment on traumatic SCI-induced locomotor dysfunction in rats
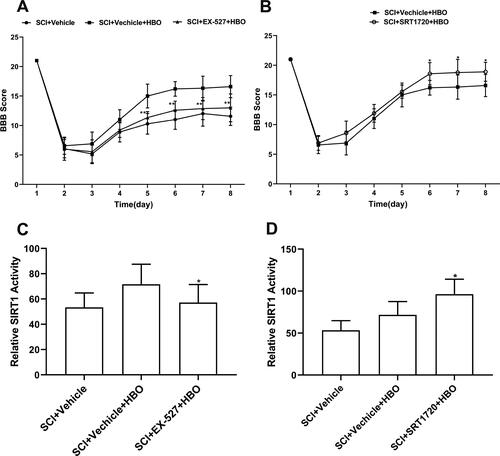
Given the modulation of series HBO treatment on SIRT1, we next determined to assess whether the SIRT1 function was involved in the effects of HBO treatment on locomotor deficit. We found that the series HBO treatment together with SIRT1 antagonist EX-527 treatment for consecutive 7 days almost blocked the effects of HBO treatment on alleviation of locomotor deficit (). However, the recovery effect of series HBO treatment on locomotor dysfunction was boosted after application of SIRT1 agonist SRT1720 for continuous 7 days (). Furthermore, SIRT1 antagonist EX-527 impeded the enhanced activity of SIRT1 following series HBO treatment in SCI rats (), while SIRT1 agonist SRT1720 for consecutive 7 days and series HBO treatment had synergistic effects on the activity of SIRT1 ().
Figure 2. Administration of SIRT1 antagonist EX-527 reversed the beneficial effects of HBO on locomotor recovery, whereas the SIRT1 agonist SRT1720 had synergic effects on the motor function with HBO therapy. (A-B) The SCI injury rats were applicated with both HBO and EX-527 (i.p.) for continuous 7 days (started at 8h postinjury) got the worse BBB scores compared with those with only administration of HBO and vehicle therapy, while the injury rats treated by HBO and intraperitoneal injection SRT1720 had the higher BBB scores versus those with only administration of HBO and vehicle therapy (n = 12 per group). (C-D) The EX-527 inhibited the enhanced activity of SIRT1 induced by HBO treatment, while SRT1720 increased effects of HBO therapy on the activity of SIRT1(n = 5 per group). *p < 0.05, **p < 0.01compared with SCI + vehicle + HBO group.
Series HBO treatment regulated the inflammatory cascade depending on the function of SIRT1
Considering that the SIRT1 can directly deacetylate and subsequently inactivate the important inflammatory transcription factor NF-κB [Citation20], which inhibits the exposure of the inflammatory cascade. The western blots showed that the acetylation of spinal cord NF-κB was significantly enhanced following the traumatic SCI injury on day 7 postinjury, while the series HBO treatment stopped the process of acetylation of NF-κB (). Next, we performed a series of Elisa assays to elucidate the role of SIRT1 in HBO treatment on inflammatory cascade. Firstly, we detected three important pro-inflammatory cytokines(TNF-α, IL-6, IL-1β) and a well-known anti-inflammatory cytokine (IL-10) in the spinal cord of rats on day 7 postinjury and following the series HBO treatment. As shown in , the enhanced expression of TNF-α, IL-6, and IL-1β in the spinal cord of SCI rats was ameliorated by HBO therapy on day 7, which was retarded by the administration of SIRT1 antagonist EX-527 treatment for consecutive 7 days (). In addition, the spinal cord of SCI injury rats that undergone the series HBO treatment together with SIRT1 agonist SRT1720 treatment for consecutive 7 days showed a decrease of TNF-α, IL-1β but not IL-6 and an abundance of IL-10 compared with that of series HBO treatment together with vehicle ().
Figure 3. SIRT1 was implicated in the inflammatory cascade induced by traumatic SCI in rats. (A)The enhanced acetylation of NF-κB in SCI rats was remarkably inhibited by HBO treatment (n = 4/per group). (B)The increase of pro-inflammatory cytokines TNF-α, IL-6 and IL-1β and decrease of anti-inflammatory cytokine IL-10 in spinal cord of SCI model rats was revered after the SCI rats was administrated with HBO (n = 5/per group). (C) The effects of HBO treatment on TNF-α, IL-6, IL-1β and IL-10 was blocked by SIRT1 antagonist EX-527 (n = 5/per group). (D) Both application of SIRT1 agonist SRT1720 and HBO promoted the effects of HBO in TNF-α, IL-1β and IL-10 but not IL-6 (n = 5/per group). ***p<0.001 compared with sham group, #p<0.05, ##p<0.01 compared with SCI group, $$p<0.01 compared with SCI + vehicle + HBO group. SCI + V(SCI + vehicle), SCI + V + H (SCI + vehicle + HBO), SCI + E + H(SCI + EX-527 + HBO), SCI + S + H (SCI + SRT1720 + HBO).
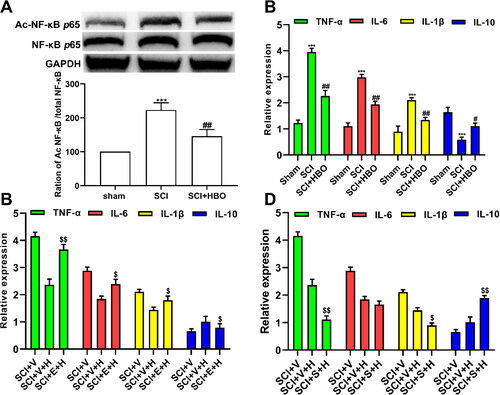
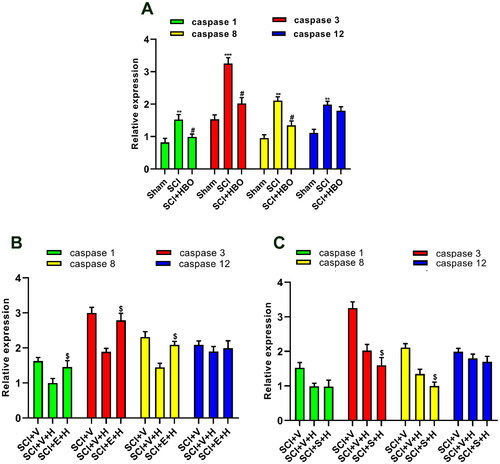
Figure 4. HBO modulated the caspase family in a SIRT1-depentent manner. (A) The increase of expression of caspase 1, caspase 3, caspase 8 but not that of caspase 12 in SCI rats was dramatically alleviated following the HBO treatment (n = 4/per group). (B) After application of HBO and SIRT1 antagonist EX-527, the decreased caspase 1, caspase 3 and caspase 8 induced by HBO treatment was reversed (n = 5/per group). (C) The effects of HBO on the increased expression of caspase 3 and caspase 8 was enhanced following intraperitoneal injection of SIRT1 agonist (n = 5/per group). **p<0.01, ***p<0.001 compared with sham group, #p<0.05, $p<0.05 compared with SCI + vehicle + HBO group. SCI + V(SCI + vehicle), SCI + V + H (SCI + vehicle + HBO), SCI + E + H(SCI + EX-527 + HBO), SCI + S + H (SCI + SRT1720 + HBO).
SIRT1 was involved in the effects of series HBO treatment on the regulation of the caspase family
It has been well-established caspase family plays a key role in the apoptosis pathway [Citation25] which mediates the process of secondary injury following the SCI [Citation26]. SIRT1 has been characterized as inhibiting apoptosis. Based on the above evidence, we conducted an Elisa assay to detect the expression of the caspase family. As shown in , series HBO treatment led to a reduction of the increased expression of caspase 1, caspase 3, and caspase 8 but not caspase 12 of the spinal cord in SCI rats on day 7 postinjury. To further evaluate the SIRT1 function in effects of series HBO treatment on the caspase family, both intraperitoneal injection SIRT1 antagonists for continuous 7 days abolished the HBO effects on caspase 1, caspase 3 and caspase 8 at day 7 postinjury (). Furthermore, the SIRT1 agonist enhanced the effects of HBO therapy on caspase 3 and caspase 8 but not caspase1 and caspase12 ().
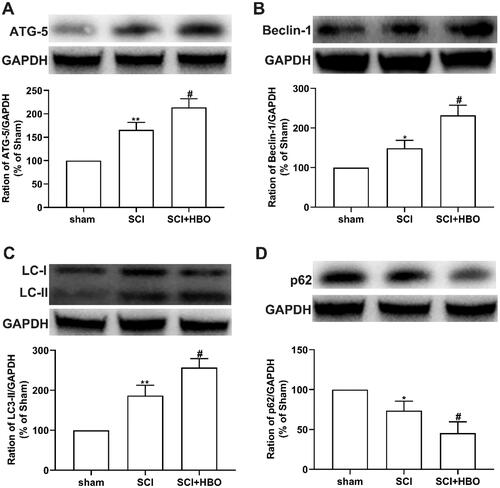
Series HBO treatment boosted the induction of autophagy postinjury in rats
Emerging shreds of evidence demonstrated that activation of SIRT1 promoted cell survival by induction of autophagy [Citation27]. Additionally, SCI injury-induced autophagy protects the damage of neurons in the spinal cord. To assess whether series HBO treatment has effects on autophagy, we performed a western blotting assay to detect the protein level of autophagy-related protein. As shown in , the expression of ATG-5, Beclin-1, and LC3-II which played a central role in the process of autophagy formation was enhanced at day 7 postinjury following the traumatic SCI injury and the series HBO treatment boosted the enhanced the effects. However, the decreased p62 implicated in the termination of autophagy in rats after SCI injury was upregulated by HBO therapy at day 7 postinjury ().
Figure 5. Induction of autophagy in spinal cord of the injury rats was enhanced following HBO treatment. (A–C) The increased expression of autophagy-related protein ATG-5, Beclin-1, LC-II following the injury was enhanced (n = 5 per/group). (D) The decreased expression of p62 was induced by SCI injury and boosted by HBO treatment (n = 5 per/group). *p<0.05,**p<0.01 compared with sham group, #p<0.05 compared with SCI group.
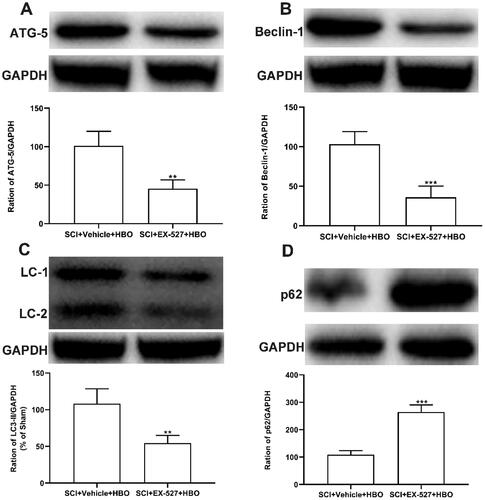
The enhanced induction of autophagy following the series HBO therapy in rats subjected to SCI was diminished by EX-527
Recently a study reported that SIRT1 improved motor nerve regeneration after peripheral nerve injury through activation of autophagy [Citation28]. These findings instigated us to further explore whether SIRT1 participated in the central nerve injury following the series HBO therapy. The immunoblot showed that the series HBO treatment promoted the increased pro-autophagy formation protein (ATG5, Beclin-1, and LC3-II), which was suppressed by administration of SIRT1 antagonist for continuous 7 days (), while expression of the reduced anti-autophagy formation protein (p62) was increased after application of SIRT1 antagonist for continuous 7 days (). These results may indicate that series HBO promoted autophagy via the promotion of the activation of activity of SIRT1 in rats subjected to SCI injury.
Figure 6. The enhanced autophagy-related protein by HBO treatment was reduced after administration of EX-527. (A-C) Intraperitoneal injection of SIRT1 antagonist EX-527 abolished the increased expression of pro-autophagy formation-related protein (ATG-5, beclin-1, and LC3-2) in SCI rats following the series HBO therapy, (D) while the level of autophagy substrate p62 was upregulated by HBO (n = 5/per group). **p<0.01, ***p<0.001 compared with SCI + vehicle + HBO group.
Discussion
In this study, we discovered that series HBO therapy hindered the decrease in expression and activity of spinal cord SIRT1 induced by traumatic SCI. The SIRT1 selective inhibitor, EX-527, partially negated the positive effects of series HBO therapy on locomotor deficits caused by SCI by inhibiting the activity of SIRT1. However, the intraperitoneal injection of SIRT1 agonist SRT1720 enhanced the functional outcome of series HBO therapy on locomotor dysfunction. Additionally, we observed that series HBO treatment increased the activity of SIRT1, leading to the suppression of the inflammatory cascade and apoptosis-related proteins. Moreover, SIRT1 promoted post-injury autophagy by upregulating the formation of autophagy proteins. Collectively, these findings suggest a novel mechanism through which series HBO therapy regulates the expression and activity of SIRT1, subsequently modulating the inflammatory cascade, apoptosis, and autophagy, ultimately improving motor dysfunction recovery.
The mechanism that series HBO mediated the inflammation cascade in a SIRT1-dependent manner
The traumatic injury exposes the spinal cord to an infiltration of inflammatory cells, which induces the overwhelming inflammatory cytokine release [Citation6]. Furthermore, the neurons and glia of the spinal cord around the injury site can propagate the inflammatory response [Citation1, Citation29]. The inflammatory cascade has been well known for its role in the process of primary to secondary damage following the SCI. Therefore, controlling the inflammatory reaction has been thought to be a versatile choice for this intractable problem. Methylprednisolone is the typical drug to control inflammatory cascade after SCI, but its side effects may restrain its function outcome [Citation30]. However, substantial evidence suggested that HBO treatment reduced the inflammatory response which may have fewer side effects [Citation31]. However, the precise mechanism underlying the anti-inflammation function of HBO deserves further investigation. A couple of studies revealed that SIRT1 can directly deacetylate histone and pivotal transcript factors such as NF-κB, resulting in the repression of the inflammatory cytokines expression [Citation20]. In the present study, we found that the enhanced acetylation of NF-κB after SCI was reduced by series HBO therapy. NF-κB signaling controls the expression of substantial inflammatory cytokines, however, SIRT1 can inhibit NF-κB signaling by deacetylation of NF-κB [Citation20]. Series HBO therapy reduced the expression of pro-inflammatory cytokines (IL-6, IL-1β, and TNF-α) and increased that of anti-inflammatory cytokine (IL-10) by activation of SIRT1. Hence, series HBO therapy activated the SIRT1 and subsequently deacetylated the NF-κB which resulted in suppressing of inflammatory response induced by SCI and improving the locomotor dysfunction. Notably, a previous study indicates that HBO inhibits the expression of CCL2 (also named MCP-1) and neutrophil infiltration [Citation12]. HBO may also block the inflammatory cell infiltration and finally inhibit the inflammatory cascade mediated by inhibitor effect of SIRT1 on CCL2. In addition, the application of SIRT1 agonist together with HBO therapy enhanced the effects of HBO on anti-inflammation and locomotor deficits, which may present a new potential strategy to alleviate SCI-induced locomotor dysfunction. It should be noted that we may conduct the HBO treatment on the traumatic SCI-induced locomotor dysfunction in SIRT1 knockout animals in the future.
The mechanism that series HBO mediated apoptosis and autophagy
Activation of pro-apoptosis signaling played a central role in the process of cell death that survived primary damage and thus controlling the apoptosis pathway can be an attractive intervention for neuroprotection [Citation32]. A vast array of proteins was involved in the process of apoptosis. Among them, the caspase family, TNF-α, and IL-1β acted as pivotal roles in pro-apoptosis. In our study, we revealed that series HBO treatment suppressed the enhanced expression of caspase 1, caspase 3, caspase 8, TNF-α and IL-1β but not caspase 12 in the spinal cord of rats subjected to traumatic SCI via regulation of SIRT1. TNF-α following binding with TNFR mediates the recruitment and activation of caspase 8 to amplify the apoptosis reaction. The activated caspase 1 participates in the cleavage and maturation of IL-1β. The above evidence indicated that series HBO repressed TNFR/caspase 8 and caspase 1/IL-1β pro-apoptosis signaling pathway through regulation of SIRT1. Recently, autophagy has been reported to promote neuronal survival via protecting motoneurons from apoptosis [Citation28]. Importantly, SIRT1 positively regulated the autophagy in different cells [Citation33, Citation34]. In the present study, we found that series HBO therapy enhanced the induced autophagy-related proteins through activation of SIRT1 which suggested that modulation of SIRT1 by series HBO may serve as neuroprotection for the survival neurons from the primary injury through enhancing the autophagy.
Conclusion
Taken together, we demonstrated that series HBO therapy regulated the SIRT1 and improved the locomotor dysfunction in rats subjected to traumatic SCI. Series HBO treatment modulated the inflammation cascade, apoptosis, and autophagy in a SIRT1-dependent manner. Herein, our study opened an avenue to a combination of HBO and SIRT agonists for the alleviation of motor dysfunction after SCI.
Authors’ contributions
Huiqiang Chen and Huai Huang designed the study. Huiqiang Chen and Ranran Xing performed the experiments. Huiqiang Chen and Xinwei Yin analyzed the data and Huai Huang wrote the manuscript. All authors read and approved the final manuscript.
Ethics approval
The experiments were performed by the guidelines of the General Hospital of Southern Theater Command Animal Ethics Committee. The protocol number is SYXK (Yue) 2020-0103.
Consent for publication
All authors consent for publication.
| Abbreviations | ||
| HBO | = | hyperbaric oxygenation |
| SCI | = | spinal cord injury |
| SIRT1 | = | sirtuin1 |
| BBB | = | Basso, Beattie Bresnahan |
| MCP-1 | = | monocyte chemoattractant protein-1 |
| NAD | = | nicotinamide adenine dinucleotide |
| NF-κB | = | nuclear factor kappa B |
| PPARγ | = | peroxisome proliferator-activated receptor γ |
| ATG-5 | = | autophagy related protein-5 |
| LC-3 | = | microtubule associated protein light chain-3 |
| IL-6 | = | interleukin-6 |
| IL-1β | = | interleukin-1β |
| IL-10 | = | interleukin-10 |
| TNF-α | = | tumor necrosis factor-α. |
Disclosure statement
There are no conflicts to declare.
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Ahuja CS, Wilson JR, Nori S, et al. Traumatic spinal cord injury. Nat Rev Dis Primers. 2017;3(1):17018. doi: 10.1038/nrdp.2017.18.
- Donovan J, Kirshblum S. Clinical trials in traumatic spinal cord injury. Neurotherapeutics. 2018;15(3):654–668. doi: 10.1007/s13311-018-0632-5.
- Beric A: post-spinal cord injury pain states. Pain. 1997;72(3):295–298.
- Hou S, Rabchevsky AG. Autonomic consequences of spinal cord injury. Compr Physiol. 2014;4(4):1419–1453. doi: 10.1002/cphy.c130045.
- Sachdeva R, Gao F, Chan CCH, et al. Cognitive function after spinal cord injury: a systematic review. Neurology. 2018;91(13):611–621. doi: 10.1212/WNL.0000000000006244.
- Orr MB, Gensel JC. Spinal cord injury scarring and inflammation: therapies targeting glial and inflammatory responses. Neurotherapeutics. 2018;15(3):541–553. doi: 10.1007/s13311-018-0631-6.
- Daly S, Thorpe M, Rockswold S, et al. Hyperbaric oxygen therapy in the treatment of acute severe traumatic brain injury: a systematic review. J Neurotrauma. 2018;35(4):623–629. doi: 10.1089/neu.2017.5225.
- Xing P, Ma K, Li L, et al. The protection effect and mechanism of hyperbaric oxygen therapy in rat brain with traumatic injury. Acta Cir Bras. 2018;33(4):341–353. doi: 10.1590/s0102-865020180040000006.
- Li HZ, Chen JF, Liu M, et al. Effect of hyperbaric oxygen on the permeability of the blood-brain barrier in rats with global cerebral ischemia/reperfusion injury. Biomed Pharmacother. 2018;108:1725–1730. doi: 10.1016/j.biopha.2018.10.025.
- Schabitz WR, Schade H, Heiland S, et al. Neuroprotection by hyperbaric oxygenation after experimental focal cerebral ischemia monitored by MRI. Stroke. 2004;35(5):1175–1179. doi: 10.1161/01.STR.0000125868.86298.8e.
- Falavigna A, Figueiro MP, Silva PGD, et al. Hyperbaric oxygen therapy after acute thoracic spinal cord injury: improvement of locomotor recovery in rats. Spine (Phila Pa 1976). 2018;43(8):E442–e447.) doi: 10.1097/BRS.0000000000002387.
- Wang Y, Li C, Gao C, et al. Effects of hyperbaric oxygen therapy on RAGE and MCP-1 expression in rats with spinal cord injury. Mol Med Rep. 2016;14(6):5619–5625. doi: 10.3892/mmr.2016.5935.
- Yu Y, Matsuyama Y, Yanase M, et al. Effects of hyperbaric oxygen on GDNF expression and apoptosis in spinal cord injury. Neuroreport. 2004;15(15):2369–2373. doi: 10.1097/00001756-200410250-00014.
- Yaman O, Yaman B, Aydin F, et al. Hyperbaric oxygen treatment in the experimental spinal cord injury model. Spine J. 2014;14(9):2184–2194. doi: 10.1016/j.spinee.2014.02.013.
- Huang L, Mehta MP, Nanda A, et al. The role of multiple hyperbaric oxygenation in expanding therapeutic windows after acute spinal cord injury in rats. J Neurosurg. 2003;99(2 Suppl):198–205. doi: 10.3171/spi.2003.99.2.0198.
- Yeo JD, Stabback S, McKenzie B. A study of the effects of hyperbaric oxygen on the experimental spinal cord injury. Med J Aust. 1977;2(5):145–147. doi: 10.5694/j.1326-5377.1977.tb99109.x.
- Herskovits AZ, Guarente L. SIRT1 in neurodevelopment and brain senescence. Neuron. 2014;81(3):471–483. doi: 10.1016/j.neuron.2014.01.028.
- Ling H, Peng L, Wang J, et al. Histone deacetylase SIRT1 targets Plk2 to regulate centriole duplication. Cell Rep. 2018;25(10):2851–2865.e2853. doi: 10.1016/j.celrep.2018.11.025.
- Nakamura K, Zhang M, Kageyama S, et al. Macrophage heme oxygenase-1-SIRT1-p53 axis regulates sterile inflammation in liver ischemia-reperfusion injury. J Hepatol. 2017;67(6):1232–1242. doi: 10.1016/j.jhep.2017.08.010.
- Kauppinen A, Suuronen T, Ojala J, et al. Antagonistic crosstalk between NF-kappaB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Signal. 2013;25(10):1939–1948. doi: 10.1016/j.cellsig.2013.06.007.
- Sathyanarayan A, Mashek MT, Mashek DG. ATGL promotes autophagy/lipophagy via SIRT1 to control hepatic lipid droplet catabolism. Cell Rep. 2017;19(1):1–9. doi: 10.1016/j.celrep.2017.03.026.
- Gomes BAQ, Silva JPB, Romeiro CFR, et al. Neuroprotective mechanisms of resveratrol in Alzheimer’s disease: role of SIRT1. Oxid Med Cell Longev. 2018;2018:8152373–8152315. doi: 10.1155/2018/8152373.
- Zhang Q, Zhang P, Qi GJ, et al. Cdk5 suppression blocks SIRT1 degradation via the ubiquitin-proteasome pathway in Parkinson’s disease models. Biochim Biophys Acta Gen Subj. 2018;1862(6):1443–1451. doi: 10.1016/j.bbagen.2018.03.021.
- Han Y, Luo H, Wang H, et al. SIRT1 induces resistance to apoptosis in human granulosa cells by activating the ERK pathway and inhibiting NF-kappaB signaling with anti-inflammatory functions. Apoptosis. 2017;22(10):1260–1272. doi: 10.1007/s10495-017-1386-y.
- Shi Y. Mechanisms of caspase activation and inhibition during apoptosis. Mol Cell. 2002;9(3):459–470. doi: 10.1016/s1097-2765(02)00482-3.
- Sobrido-Camean D, Barreiro-Iglesias A. Role of caspase-8 and fas in cell death after spinal cord injury. Front Mol Neurosci. 2018;11:101. doi: 10.3389/fnmol.2018.00101.
- Lee IH, Cao L, Mostoslavsky R, et al. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci USA. 2008;105(9):3374–3379. doi: 10.1073/pnas.0712145105.
- Romeo-Guitart D, Leiva-Rodriguez T, Fores J, et al. Improved motor nerve regeneration by SIRT1/Hif1a-mediated autophagy. Cells. 2019;8(11):1354. doi: 10.3390/cells8111354.
- Okada S, Hara M, Kobayakawa K, et al. Astrocyte reactivity and astrogliosis after spinal cord injury. Neurosci Res. 2018;126:39–43. doi: 10.1016/j.neures.2017.10.004.
- Bracken MB, Shepard MJ, Collins WF, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the second national acute spinal cord injury study. N Engl J Med. 1990;322(20):1405–1411. doi: 10.1056/NEJM199005173222001.
- Patel NP, Huang JH. Hyperbaric oxygen therapy of spinal cord injury. Med Gas Res. 2017;7(2):133–143. doi: 10.4103/2045-9912.208520.
- Tran AP, Warren PM, Silver J. The biology of regeneration failure and success after spinal cord injury. Physiol Rev. 2018;98(2):881–917. doi: 10.1152/physrev.00017.2017.
- Qiu G, Li X, Che X, et al. SIRT1 is a regulator of autophagy: implications in gastric cancer progression and treatment. FEBS Lett. 2015;589(16):2034–2042. doi: 10.1016/j.febslet.2015.05.042.
- Ng F, Tang BL. Sirtuins’ modulation of autophagy. J Cell Physiol. 2013;228(12):2262–2270. doi: 10.1002/jcp.24399.

