Abstract
Aim
Lambl’s excrescences are mobile, thin, fibrinous connective tissue strands typically found on left-sided cardiac values. Migraine is positively associated with structural cardiac anomalies. However, it remains unclear whether Lambl’s excrescences are associated with migraine.
Methods
Retrospective review of 182 inpatients with Lambl’s excrescences confirmed by transesophageal echocardiogram in Chinese PLA General Hospital since January 2010. Among them, those with isolated Lambl’s excrescences presented with migraine-like headache were included. We collected information on the demographics and clinical profiles of all participants, and performed follow-up visits.
Results
A total of 8 patients presented with migraine-like headache among 15 patients with isolated Lambl’s excrescences. They included 2 men and 6 women, with an average age of 44.63 ± 12.24 years. Among these patients, 3 had visual aura, and 6 manifested infarct-like lesions on magnetic resonance imaging, of which 2 developed lesions after first visit. During follow-up, 4 patients suffering from intervention for Lambl’s excrescences dramatically reduced headache recurrence compared to the other 4 patients only receiving migraine preventive medications.
Conclusions
This study supports the hypothesis that microemboli from isolated Lambl’s excrescences could cause migraine-like headache. And intervention for Lambl’s excrescences may be crucial for preventing headache recurrence.
STRENGTHS AND LIMITATIONS OF THIS STUDY
This study supports the hypothesis that microemboli from isolated Lambl’s excrescences could cause migraine-like headache.
The small sample size study fails to make management recommendations.
Introduction
Lambl’s excrescences (LEs), first described by a Bohemian physician-scientist Vilem Dusan Lambl in 1856, are typically thin, mobile, fibrinous valvular strands that develop at the valvular coaptation sites of the heart. On an echocardiogram, valvular strands have been found mostly on both mitral and aortic valves with a diameter of approximate 1 mm (mm) and a length of up to 10 mm. Histologically, these are acellular, composed of a central core of elastic connective tissue and a single layer of endothelium. Etiologically, they may be related to endocardial wear in the contact margins of a valve caused by exposure to the high stress [Citation1,Citation2]. A 2-year retrospective study showed that the prevalence of valvular strands was 5.5% in a general patient population referred for a transesophageal echocardiogram (TEE) [Citation3]. Although the prevalence increases with age, the incidence of valvular strands peaks at age group from 61 to 70 years (0.94%) [Citation2].
The clinical significance of LEs is not yet clear and is generally considered to be a physiological finding that is not associated with cardiovascular events [Citation4,Citation5]. However, some case reports have suggested that LEs were the potential etiology of embolic stroke [Citation6–8]. Recently, Robertson et al. reported a 10-year-old boy who presented with acute right-sided hemiplegia. Imaging examination found multifocal embolic strokes of various ages. Extensive stroke workup was unrevealing, aside from the presence of LEs on the mitral and aortic valves [Citation1].
Migraine is a common chronic, disabling, and neurovascular brain disorder. The pathophysiology is unclear. Ischemic heart disease and ischemic stroke are common comorbidities of migraine [Citation9]. Results from numerous observational studies have suggested migraine is positively associated with cardiac and pulmonary right-to-left shunt (RLS) as well as structural cardiac anomalies in the absence of shunt, including patent foramen ovale (PFO), atrial septal defects, pulmonary arteriovenous malformations, mitral valve prolapse, atrial septal aneurysm, congenital heart disease, and so on [Citation10,Citation11]. Interestingly, our team found that migraine-like headache occurred in two patients with LEs [Citation12]. However, the underlying mechanism remains unclear. Thus, on this basis, we conducted a retrospective study to investigate the association between isolated LEs and migraine-like headache.
Materials and methods
Patient and public involvement
Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Participants
Inclusion criteria: Inpatients with isolated LEs who presented with migraine-like headache in the Chinese People’s Liberation Army (PLA) General Hospital since January 2010. Exclusion criteria: (1) with heart comorbidities: at least one heart disease, such as infective endocarditis (IE), congenital heart disease (CHD), coronary artery disease (CAD), myocardial infarction (MI), heart failure (HF), and PFO; (2) without migraine-like headache; (3) with acute headache attributed to ischaemic stroke (6.1.1.1 in the third edition of the International Classification of Headache Disorders, ICHD-3).
Measurements
LEs were diagnosed by experienced echocardiologists through TEE. Important and challenging differential diagnosis includes fibroelastomas, vegetations and thrombi [Citation13–15]. The type of headache was diagnosed by independent, trained neurologists in the international headache center according to ICHD-3 [Citation16]. The intensity of headache is determined according to the visual analogue scale (VAS). Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria were used to classify the cause of ischemic strokes [Citation17]. The common pathogeneses of ischemic stroke were evaluated according to nationwide studies of stroke [Citation18,Citation19] and risk factors of ischemic stroke in younger adults [Citation20], including hypertension, dyslipidaemia, diabetes, smoking, alcohol consumption, atrial fibrillation, protein C and S deficiency, antithrombin III deficiency, metabolic syndrome, and use of contraception containing estrogen, etc.
Data collection and follow-up
We retrospectively collected the data of demographic, clinical, radiological, laboratory test, diagnosis, and treatment of patients. During the follow-up, we recorded the patients’ information in detail, such as whether there was a recurrence of headache, whether insist on taking medications for headache, headache was improved or aggravated, is there any intervention on LEs, and is there any new infarct-like lesions in magnetic resonance imaging (MRI), etc.
Literature review
We searched PubMed database for articles published in English on July 1, 2022, using the terms ‘Lambl excrescences,’ ‘Lambl’s excrescences’, ‘valvular strands’, ‘valve strands’, ‘headache’, and ‘migraine’. We also inspected reference lists of articles and reviews.
Statistical analysis
The IBM SPSS software, version 26.0 was used for the statistical analysis. The age of the subjects was presented as the mean ± standard deviation (SD).
Results
Baseline demographic and clinical characteristics
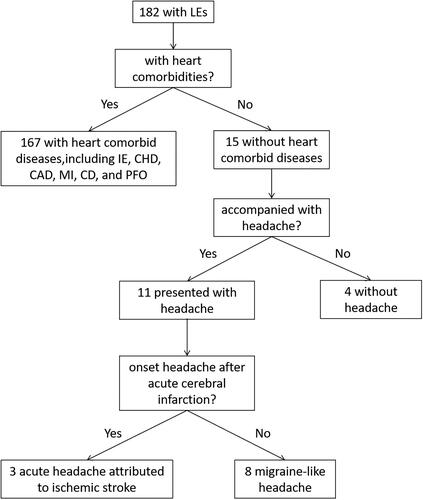
A total of 182 inpatients were diagnosed with LEs in our hospital from January 2010 to present. 1A total of 67 patients comorbid with at least one heart disease were excluded. Among the remaining 15 patients with isolated LEs, 7 of them were further excluded: 4 patients were diagnosed with embolic stroke potentially related to LEs, and the other 3 patients suffered from acute embolic stroke accompanied with headache, the onset of whose headache is urgent and closely related to the time of stroke attack. Finally, only 8 patients who presented with migraine-like headache were included in this study: 7 of them found LEs when screening the causes of headache and for the remaining 1 patient, LEs were identified during investigation for the cause of cerebral infarction after intermittent headache. The flow chart of the inclusion and exclusion process was shown in .
Figure 1. Flow chart in screening patients of headache associated with LEs. IE, infective endocarditis; CHD, congenital heart disease; CAD, coronary artery disease; MI, myocardial infarction; HF, heart failure; PFO, patent foramen ovale.
These 8 patients (2 men, 6 women) aged from 23 to 56 years old, with an average age of 44.63 ± 12.24 years at time of diagnosis. Some of them had headache disorders for years but never got a definitive diagnosis. Except case 2 was diagnosed with hypertension, no common risk factors for stroke were identified in these patients. They had unremarkable prior medical history, and all denied family history of headache. The characteristics of headache are summarized in .
Table 1. Characteristics of headache associated with LEs in 9 patients.
The results of carotid ultrasonography and Cranial magnetic resonance angiography (MRA) failed to reveal arterial abnormalities. Brain MRI showed no significant abnormalities in 4 patients (case 1/3/4/6). However, multiple Infarct-like lesions were confirmed on MRI in other 4 patients (case 2/5/7/8), including 1 patient (case 2) with migraine with aura (MA), but the visual aura was not consistent with the location of lesions. Case 8 presented with an acute onset of high-grade right-sided hemiparesis and dysarthric speech half a year after intermittent headache, which was diagnosed with acute cerebral infarction. Diffusion restriction was showed on MRI diffusion-weighted imaging (DWI) sequence in the corpus callosum and right temporal-parietal lobe, and multiple, small hyperintensities could be seen in white matter of the left temporal and occipital lobe. All these 8 patients had no family history of headache, and none of them had positive signs of neurological defects except case 8. TEE revealed at least one filiform, mobile mass attached to the aortic valve—a finding consistent with the presence of LEs () and coagulation function were normal in all patients ().
Figure 2. TEE revealed a mobile, thin, fibrous cord-like echo attached to the edge of the aortic valve (case 5).
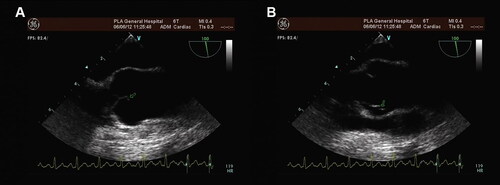
Table 2. Partial auxiliary examinations, treatment, and follow-up of 9 patients.
Follow-up
Follow-up of all patients for up to 12 years showed that LEs were intervened in 4 patients with infarct (case 2/5/7/8), including 1 underwent successful open-heart surgery for surgical excision of LEs (the histopathological results support the diagnosis of LEs), 1 was discharged on a regimen of warfarin (3.75 mg once daily) and sodium valproate (500 mg once daily), 1 was treated with aspirin (100 mg once daily) and flunarizine (10 mg once daily), and 1 just took aspirin (100 mg once daily). There was rare or no recurrence of migraine-like headache. Among the other 4 patients (case 1/3/4/6) who received only drugs for the prophylaxis of migraine without intervention for LEs, 1 patient switched to Chinese proprietary medicine due to ineffective preventive medications. The headache symptoms of the other 3 patients were improved, but it was easy to relapse after withdrawal. White matter lesions were found in 2 patients after re-examination of cranial MRI, whose previous MRI results were negative (case 3 and 4) ().
Literature review
After searching, only 1 patient with migraine-like headache associated with Lambl’s excrescences has been reported. In 2015, Davogustto G et al. described a female aged 65–70 years old and presented with migrainous headache. Transthoracic echocardiography (TTE) showed a prominent LE on the right coronary cusp protruding into the ventricular surface of the aortic valve [Citation21]. Her headache characteristics are shown as case 9 in . MRI and computed tomography (CT) showed a previous ischemic stroke. A CT angiogram excluded significant stenosis of the intra- or extracranial vasculature. Migrainous headache relief when given acute medication therapy of migraine such as dihydroergotamine and divalproex sodium. The patient did not have a recurrence of migraine or ischaemic stroke with the administration of propranolol and aspirin for the prophylaxis ().
Discussion
In this study, 8 of 15 patients with isolated LEs developed migraine-like headache, which was significantly higher than the prevalence of migraine in a population-based door-to-door survey in mainland of China (9.3%) [Citation22], although some patients did not completely meet diagnosis criteria for migraine. A TEE-based study showed that LEs was present in 11 of 51 consecutive outpatients with MA [Citation23]. The finding was higher than the prevalence of LEs in a general population (5.5%) [Citation3]. Women comprised 75% of the patients we investigated following migraine-like headache, which is similar to the susceptibility of men and women to migraine [Citation24].
Lambl’s excrescences, appear to be wear-and-tear lesion, occur at and around the coaptation site of left-sided cardiac valves. Different studies had different conclusions comparing the prevalence of aortic and mitral valve [Citation2,Citation25,Citation26]. In our study, LEs were all found on the aortic valve, which may be due to higher pressures on the aortic valve compared to mitral valve. Interestingly, embolic events such as ischemic stroke, acute coronary syndrome, and peripheral embolism were more commonly observed in association with LEs on aortic valves [Citation27]. A TEE-based case-control study found a link between cerebral ischemia and LEs across gender and race-ethnic subgroups, which was more pronounced in younger than older adults. Strands on both the mitral (odds ratio (OR) = 3.5; 95% confidence interval (CI) = 1.5 to 7.9) and aortic (OR = 3.7; 95% CI = 1.1 to 11.9) valve were associated with ischemic stroke, whereas the number and length of strands were not [Citation28]. Aziz et al. [Citation29] reported a 61-year-old woman presented with recurrent ischemic strokes, whose TEE results showed LEs on the aortic valve. She underwent successful open-heart surgery for debridement of LEs after all potential causes of stroke were ruled out. The histopathologic diagnosis confirmed LEs. The patient did not have any cerebrovascular embolic event after surgery. Many similar cases have been reported, but the pathological mechanism behind it has not been clarified. It is speculate that due to the high stress leading to shear force and trauma on the heart valves, the endocardium on the valvular cusps at the coaptation site may occasionally be torn, followed by fibrin deposition and small thrombi formation on the injury [Citation15]. LEs may break off and cause distal organs embolism.
Some clinic-based studies have shown that migraine, especially MA was associated with cardiovascular diseases and ischemic stroke. For example, a Danish nationwide population based matched cohort study showed that migraine was associated with increased risks of myocardial infarction, ischaemic stroke, haemorrhagic stroke, venous thromboembolism, and atrial fibrillation (AF) or atrial flutter [Citation30]. In ICHD-3, 1.4.3 migrainous infarction was listed as one of the complications of migraine, which is rare but well documented. According to the prospective, population-based Atherosclerosis Risk in Communities (ARIC) study, migraine with visual aura was significantly associated with cardioembolic stroke (hazard ratio (HR) 3.7, 95% CI 1.6–8.7, p = 0.003), but not of lacunar or nonlacunar thrombotic stroke [Citation31]. A significant amount of brain MRI studies have linked migraine with white matter changes and subclinical infarct-like lesions [Citation32,Citation33]. In our study, 6 out of 8 patients developed white matter lesions on MRI.
This study suggested that headache associated with isolated LEs conforms to almost all the characteristics of migraine. And our results indicated that the recurrence of headache in patients suffering from intervention for LEs significantly decreased than that in patients only taking preventive medications. To our knowledge little attention has been paid on the link between migraine-like headache and isolated LEs. In 2015, Davogustto G and colleagues [Citation21] showed in their study a woman with isolated LEs who developed late-onset migrainous headaches. After the combined treatment of antiplatelet and migraine preventive drugs, the headache did not appear again. So there seemed to be an association between isolated LEs and migraine-like headache. However, the underlying mechanism is unknown.
Numerous studies have reported that migraine is closely associated with vascular system abnormalities. Some showed that right-to-left shunt might play a role in the etiology of migraine. Investigations using contrast-enhanced transcranial Doppler (TCD) in nine Chinese hospitals demonstrated the prevalence of RLS was 63.8% and 39.9% in MA group and migraine without aura (MO) group, respectively, significantly higher than that of the healthy group (29.4%, p < 0.001; p < 0.001) [Citation34]. It has been postulated that vasoactive substances such as serotonin in venous circulation that can trigger migraine are filtered or inactivated in the alveolar capillary bed, and RLS allowed such substances to bypass this filter and thus reach the cerebral circulation. Moreover, a microembolus in the venous circulation can pass through RLS and directly into the cerebral arterial system, causing focal areas of hypoperfusion, which in turn triggered cortical spreading depression (CSD) [Citation35]. Most cells in a brain area underwent near-complete membrane depolarization during CSD, which was the likely underlying mechanism of migraine aura, and then CSD activated meningeal nociceptors, the first step in the activation of the trigeminovascular pathway resulting in migraine [Citation9]. For example, paradoxical gas embolism may explain the auras occurred after dives in divers suffered decompression illness with no history of migraine [Citation35]. In a mouse model, Nozari et al. [Citation36] readily evoked CSD by introducing microemboli into the carotid artery. This hypothesis may also explain why migraine attacks can be relieved by the use of antiplatelets or anticoagulants. A lot of attention has been placed on investigating the link between PFO and migraine. Observational studies have shown migraine is more prevalent in subjects with PFO and vice versa [Citation37]. Two prospective, randomized trials used the Amplatzer PFO Occluder to assess the efficacy of percutaneous device closure as a therapy for migraine with or without aura [Citation38]. Their results demonstrated that PFO closure significantly reduced the mean number of monthly migraine days, monthly migraine attacks (although not up to 50%), and resulted in a greater number of subjects who experienced complete migraine cessation compared to medical treatment only.
In addition to a cardiac RLS, a pulmonary RLS is also associated with migraine. Wong et al. [Citation39] summarized the clinical features of 20 patients diagnosed with idiopathic pulmonary arteriovenous malformation (PAVM). One of the most common symptoms reported was migraine (30% of patients), second only to dyspnoea. Bellofiore et al. [Citation40] reported a 37-year-old woman, with a history of chronic MA, was referred to the emergency room due to dysarthria and left faciobrachial paresis. Brain MRI was normal, but chest CT showed a large complex PAVM. After occlusion of vascular malformations with transcatheter embolization, her clinical status significantly improved soon and no relapse of migraine was reported. This is compatible with a 10-year retrospective study which showed a significant benefit of embolization of PAVMs in improving migraine: the overall prevalence of migraine decreased from 45.2% before to 34.5% after embolization (p = 0.01); the prevalence of MA decreased from 33.3% before to 19.0% after embolization (p = 0.002); the severity of headache attacks decreased in patients who still had migraine (p = 0.15) or MA after embolization (p = 0.11) [Citation41]. A similar situation also occurred in patients with pulmonary arteriovenous fistula (PAVF) [Citation42].
However, some studies focused on the association between migraine and non-right-to-left shunt abnormalities. Ceuster et al. [Citation43] reported a 52-year-old woman with MA, who was diagnosed as cerebral infarction because of transient numbness of the left arm and the lips on the left side. TTE was performed in order to find the potential cardiac sources of embolus and a large myxoma of the left atrial wall was found. After surgical removal of the myxoma, she was free of migraine symptoms. The author reviewed 7 cardiac myxoma associated migraine patients and found that the migraine ceased after removal of the myxoma in all cases, which implies a causal relationship. The author postulated that microemboli triggered CSD, and thus led to MA. Similar to this study, Dong Hoon Shin et al. [Citation44] described a 19-year-old female with recurrent MA for 7 years. Multiple stroke lesions were showed on MRI in the posterior circulation. Moreover, angiography revealed a pseudoaneurysm (1.9 × 1.4 cm) originating from the left vertebral artery. Multiple microembolic signals were repeatedly observed in the basilar artery using transcranial Doppler monitoring. Interestingly and surprisingly, the microembolic signals and her typical migrainous symptoms disappeared simultaneously with removal of the pseudoaneurysm. Several studies indicate a significant association of MA and atrial fibrillation. Ablation therapy for atrial fibrillation in patients with migraine might reduce migraine attacks, implies that migraine may be secondary to atrial fibrillation-associated microemboli [Citation45].
Therefore, we proposed that migraine-like headache associated with LEs may be attributed to cardiac microemboli, which was supported by our patients’ clinical manifestations and the therapeutic effect of intervention for LEs. But clinical follow-up found that the association between migraine and ischemic stroke among isolated LEs patients, was mysterious and complex. Migraine is almost certainly a risk factor for ischemic stroke. Theoretically, the time sequence may also be reversed, that is first cerebral ischemia, which induces CSD and in turn triggers aura and migraine [Citation9]. More research is needed to better understand how isolated LEs, migraine, and ischemic stroke interact with each other. Migraineurs with suspected cryptogenic stroke should be screened for LEs as a priority, similar to PFO and AF. Meanwhile, it was important to distinguish migraine-like headache as a primary headache from a secondary headache originating from other disorders that can by themselves lead to ischemic stroke.
Limitations
This study has several limitations. First, we only collected the data from a tertiary hospital in Beijing, which may limit the broad applicability of our findings. Second, our hypothesis comes from clinical observation study and lacks direct and definitive pathophysiology evidence. Third, the small sample size study fails to make management recommendations. A well-designed, multicenter prospective study comparing migraine-like headache pre- and post-intervention for LEs will help guide best practice in treatment.
Conclusions
The current study illustrated that migraine-like headache may be associated with microemboli from isolated LEs. And intervention for LEs may play a crucial role in preventing headache recurrence compared to only drug prevention. Patients with migraine and isolated LEs should be closely followed up.
Authors’ contributions
Dr. WX was responsible for reviewing the literature and writing the manuscript. Dr. XW, RL, ZJ and SM were responsible for data collection. Dr. YL and CY were responsible for headache diagnosis. Dr. CL and HZ was responsible for the follow-up of patients. Dr. SY and RL were the principal investigators who were responsible for study design, data analysis and interpretation, and revision of the manuscript. As co-corresponding author, Dr. SY and RL had full access to all the data in the study and had final responsibility for the decision to submit for publication. All authors read and approved the final manuscript.
Ethics approval
This study was approved by the Ethics Committee of Chinese PLA General Hospital.
| Abbreviations | ||
| Les | = | Lambl’s excrescences |
| TEE | = | transesophageal echocardiogram |
| PFO | = | patent foramen ovale |
| RLS | = | right-to-left shunt |
| ICHD-3 | = | International Classification of Headache Disorders, 3rd edition |
| MO | = | migraine without aura |
| MA | = | migraine with aura |
| CSD | = | cortical spreading depression |
| PAVM | = | pulmonary arteriovenous malformation. |
Acknowledgements
The authors gratefully acknowledge the doctors and patients who participated in this study.
Disclosure statement
The authors declare that they have no competing interests.
Data availability statement
The data used and analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Robertson DM, Wright MA, Ostrander B, et al. Child neurology: case report of lambl excrescences in a pediatric patient with multifocal strokes. Neurology. 2022;99(2):73–76. doi: 10.1212/WNL.0000000000200747.
- Leitman M, Tyomkin V, Peleg E, et al. Clinical significance and prevalence of valvular strands during routine echo examinations. Eur Heart J Cardiovasc Imaging. 2014;15(11):1226–1230. doi: 10.1093/ehjci/jeu110.
- Freedberg RS, Goodkin GM, Perez JL, et al. Valve strands are strongly associated with systemic embolization: a transesophageal echocardiographic study. J Am Coll Cardiol. 1995;26(7):1709–1712. doi: 10.1016/0735-1097(95)00394-0.
- Roldan CA, Schevchuck O, Tolstrup K, et al. Lambl’s excrescences: association with cerebrovascular disease and pathogenesis. Cerebrovasc Dis. 2015;40(1-2):18–27. doi: 10.1159/000381906.
- Zuo S, Bo X, He L, et al. Lambl’s excrescence and the safety of radiofrequency ablation for atrial fibrillation. Pacing Clin Electrophysiol. 2022;45(7):821–825. doi: 10.1111/pace.14518.
- Aggarwal A, Leavitt BJ. Images in clinical medicine. Giant lambl’s excrescences. N Engl J Med. 2003;349(25):e24. doi: 10.1056/ENEJMicm010900.
- Nighoghossian N, Derex L, Loire R, et al. Giant lambl excrescences. An unusual source of cerebral embolism. Arch Neurol. 1997;54(1):41–44. doi: 10.1001/archneur.1997.00550130027011.
- Kalavakunta JK, Peddi P, Bantu V, et al. Lambl’s excrescences: a rare cause of stroke. J Heart Valve Dis. 2010;19(5):669–670.
- Ferrari MD, Goadsby PJ, Burstein R, et al. Migraine. Nat Rev Dis Primers. 2022;8(1):2. doi: 10.1038/s41572-021-00328-4.
- Schwedt TJ. The migraine association with cardiac anomalies, cardiovascular disease, and stroke. Neurol Clin. 2009;27(2):513–523. doi: 10.1016/j.ncl.2008.11.006.
- Jesurum JT, Fuller CJ, Velez CA, et al. Migraineurs with patent foramen ovale have larger right-to-left shunt despite similar atrial septal characteristics. J Headache Pain. 2007;8(4):209–216. doi: 10.1007/s10194-007-0396-5.
- Liu RZ, Yu SY, Li Y. Migraine-Like headache and ischemic strokes in two patients with lambl’s excrescences. Chin Med J (Engl). 2012;125(18):3346–3348.
- Gowda RM, Khan IA, Nair CK, et al. Cardiac papillary fibroelastoma: a comprehensive analysis of 725 cases. Am Heart J. 2003;146(3):404–410. doi: 10.1016/S0002-8703(03)00249-7.
- Menzel T, Mohr-Kahaly S, Arnold KJ, et al. Detection of strands in native aortic valves by transesophageal echocardiography. Am J Cardiol. 1997;79(11):1549–1552. doi: 10.1016/s0002-9149(97)00193-8.
- Jaffe W, Figueredo VM. An example of lambl’s excrescences by transesophageal echocardiogram: a commonly misinterpreted lesion. Echocardiography. 2007;24(10):1086–1089. doi: 10.1111/j.1540-8175.2007.00533.x.
- Headache Classification Committee of the International Headache Society. (Ihs) the international classification of headache disorders, 3rd edition. Cephalalgia. 2018;38(1):1–211. doi: 10.1177/0333102417738202.
- Adams HP, Jr., Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. Toast. Trial of org 10172 in acute stroke treatment. Stroke. 1993;24(1):35–41. doi: 10.1161/01.str.24.1.35.
- Wu S, Wu B, Liu M, et al. Stroke in China: advances and challenges in epidemiology, prevention, and management. Lancet Neurol. 2019;18(4):394–405. doi: 10.1016/S1474-4422(18)30500-3.
- Gan Y, Jiang H, Room R, et al. Prevalence and risk factors associated with stroke in China: a nationwide survey of 726,451 adults. Eur J Prev Cardiol. 2021;28(12):e6–e10. doi: 10.1177/2047487320902324.
- George MG. Risk factors for ischemic stroke in younger adults: a focused update. Stroke. 2020;51(3):729–735. doi: 10.1161/STROKEAHA.119.024156.
- Davogustto G, Fernando RR, Loghin C. Lambl’s excrescence, migrainous headaches, and "tiger stripes": puzzling findings in one patient. Tex Heart Inst J. 2015;42(1):70–72. doi: 10.14503/THIJ-13-3808.
- Yu S, Liu R, Zhao G, et al. The prevalence and burden of primary headaches in China: a population-based door-to-door survey. Headache. 2012;52(4):582–591. doi: 10.1111/j.1526-4610.2011.02061.x.
- Zhai YN, Li Y, Wei LQ, et al. Incidences of aortic and mitral valve strands in patients with migraine with aura. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2015;37(2):147–151. doi: 10.3881/j.issn.1000-503X.2015.02.003.
- Collaborators GBDH. Global, regional, and national burden of migraine and Tension-Type headache, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2018;17(11):954–976. doi: 10.1016/S1474-4422(18)30322-3.
- Homma S, Di Tullio MR, Sciacca RR, et al. Effect of aspirin and warfarin therapy in stroke patients with valvular strands. Stroke. 2004;35(6):1436–1442. doi: 10.1161/01.STR.0000126100.53682.a0.
- Roldan CA, Shively BK, Crawford MH. Valve excrescences: prevalence, evolution and risk for cardioembolism. J Am Coll Cardiol. 1997;30(5):1308–1314. doi: 10.1016/s0735-1097(97)00315-x.
- Chu A, Aung TT, Sahalon H, et al. Lambl’s excrescence associated with cryptogenic stroke: a case report and literature review. Am J Case Rep. 2015;16:876–881. doi: 10.12659/ajcr.895456.
- Roberts JK, Omarali I, Di Tullio MR, et al. Valvular strands and cerebral ischemia. Effect of demographics and strand characteristics. Stroke. 1997;28(11):2185–2188. doi: 10.1161/01.str.28.11.2185.
- Aziz F, Baciewicz FA. Jr. Lambl’s excrescences: review and recommendations. Tex Heart Inst J. 2007;34(3):366–368.
- Adelborg K, Szépligeti SK, Holland-Bill L, et al. Migraine and risk of cardiovascular diseases: danish population based matched cohort study. BMJ. 2018;360:k96. doi: 10.1136/bmj.k96.
- Androulakis XM, Kodumuri N, Giamberardino LD, et al. Ischemic stroke subtypes and migraine with visual aura in the aric study. Neurology. 2016;87(24):2527–2532. doi: 10.1212/WNL.0000000000003428.
- Tana C, Tafuri E, Tana M, et al. New insights into the cardiovascular risk of migraine and the role of white matter hyperintensities: is gold all that glitters? J Headache Pain. 2013;14(1):9. doi: 10.1186/1129-2377-14-9.
- Bashir A, Lipton RB, Ashina S, et al. Migraine and structural changes in the brain: a systematic review and meta-analysis. Neurology. 2013;81(14):1260–1268. doi: 10.1212/WNL.0b013e3182a6cb32.
- Wang SB, Liu KD, Yang Y, et al. Prevalence and extent of right-to-left shunt on Contrast-Enhanced transcranial doppler in chinese patients with migraine in a multicentre case-Control study. Cephalalgia. 2018;38(4):690–696. doi: 10.1177/0333102417708203.
- Wilmshurst P, Nightingale S. Relationship between migraine and cardiac and pulmonary right-to-left shunts. Clin Sci (Lond). 2001;100(2):215–220. doi: 10.1042/CS20000231.
- Nozari A, Dilekoz E, Sukhotinsky I, et al. Microemboli may link spreading depression, migraine aura, and patent foramen ovale. Ann Neurol. 2010;67(2):221–229. doi: 10.1002/ana.21871.
- Liu K, Wang BZ, Hao Y, et al. The correlation between migraine and patent foramen ovale. Front Neurol. 2020;11:543485. doi: 10.3389/fneur.2020.543485.
- Mojadidi MK, Kumar P, Mahmoud AN, et al. Pooled analysis of PFO occluder device trials in patients with PFO and migraine. J Am Coll Cardiol. 2021;77(6):667–676. doi: 10.1016/j.jacc.2020.11.068.
- Wong HH, Chan RP, Klatt R, et al. Idiopathic pulmonary arteriovenous malformations: clinical and imaging characteristics. Eur Respir J. 2011;38(2):368–375. doi: 10.1183/09031936.00075110.
- Bellofiore B, Santoro G, D’Alto M, et al. Chronic migraine and transient ischemic attack due to isolated pulmonary arteriovenous malformation successfully treated with transcatheter embolization. J Cardiovasc Med (Hagerstown). 2011;12(3):215–217. doi: 10.2459/JCM.0b013e32833b9aea.
- Post MC, Thijs V, Schonewille WJ, et al. Embolization of pulmonary arteriovenous malformations and decrease in prevalence of migraine. Neurology. 2006;66(2):202–205. doi: 10.1212/01.wnl.0000194257.75559.b0.
- Yang J, Huang X, Yin S, et al. A 38-year-old woman with global aphasia and migraine. Chest. 2018;153(5):e119–e22. doi: 10.1016/j.chest.2017.12.003.
- de Ceuster L, van Diepen T, Koehler PJ. Migraine with aura triggered by cardiac myxoma: case report and literature review. Cephalalgia. 2010;30(11):1396–1399. doi: 10.1177/0333102410378928.
- Shin DH, Lim TS, Yong SW, et al. Posterior circulation embolism as a potential mechanism for migraine with aura. Cephalalgia. 2012;32(6):497–499. doi: 10.1177/0333102412441722.
- Scutelnic A, Mattle HP, Branca M, et al. Migraine and atrial fibrillation: a systematic review. Eur J Neurol. 2022;29(3):910–920. doi: 10.1111/ene.15198.

