Summary
The Argentine ant, Linepithema humile (Mayr, 1868), is a highly invasive species with serious detrimental consequences to native fauna and flora in invaded territories. Native to South-Central South America, it was first reported from continental Africa in 1893 but, to date, had never been recorded from the Malagasy region (encompassing Madagascar, Comoros, Seychelles, and Mascarene archipelago). Here, we report the first record of this invasive species on the island of La Réunion, a French overseas territory part of the Mascarene archipelago. The species was identified using both morphological and molecular analysis. We discuss potential ecological consequences of the presence of this ant on the insular ecosystems of La Réunion.
Résumé
Premier signalement de la fourmi d'Argentine, Linepithema humile (Mayr, 1868) (Hymenoptera : Formicidae), pour la région Malgache. La fourmi d’Argentine, Linepithema humile (Mayr, 1868), est une espèce hautement invasive générant des conséquences considérables sur la faune et la flore des territoires envahis. Originaire du Centre-Sud de l’Amérique du Sud, cette espèce a été découverte en Afrique continentale dès 1893 mais n’avait, jusqu’à présent, pas été signalée de la région Malgache (comprenant Madagascar, les Comores, Seychelles et l’archipel des Mascareignes). Nous rapportons ici la première découverte de cette espèce invasive sur l’ile de La Réunion, territoire français d’outre-mer faisant partie des Mascareignes. L’identité de l’espèce a été confirmée grâce à des analyses moléculaires et morphologiques. Nous discutons ensuite des conséquences écologiques potentielles de la présence de cette espèce sur les écosystèmes insulaires de La Réunion.
Invasive species are a threat to ecosystems worldwide as they can disrupt ecological equilibrium with detrimental consequences to native biodiversity and ecosystem functions. Biological invasions can indeed result in irreversible community shifts through alterations of the composition or abundance of native organisms, sometimes leading to species extinctions (Bellard et al. Citation2016). This can result in the modification of ecosystem functions and thus impact provision of ecosystem services, with major economic and health costs to human societies (Kumschick et al. Citation2015; Ogden et al. Citation2019; Diagne et al. Citation2021). Early detection of invasive species can however greatly reduce ecological and societal consequences because of the damage being less extensive and control easier when populations are still small (Mehta et al. Citation2007).
Among invasive species, the Argentine ant, Linepithema humile (Mayr, 1868) (Hymenoptera: Formicidae), is known as one of the 100 world worst invaders by the International Union for Conservation of Nature (IUCN) (Lowe et al. Citation2000; Global Invasive Species Database (GISD) Citation2023). Native to south-central Latin America, this typical “tramp” species has spread through human mediated transports to North America (earliest report in 1891), Asia (1944), Europe (1890), continental Africa (1893) and also occurs in many oceanic islands (Holway et al. Citation2002; Wetterer et al. Citation2009; Guénard et al. Citation2017). Although it thrives in Mediterranean climates alike those of its native area, it is well established in (sub)tropical zones such as Hawaii where environmental gradients provide suitable abiotic conditions (Wetterer et al. Citation2009). Furthermore, this small non-polymorphic ant exhibits specific traits explaining its invasive success (Tsutsui & Suarez Citation2003). After human-mediated long-distance jump dispersals, the Argentine ant is capable of establishing nests in a broad range of substrates. These nests can be ephemeral as rapid relocation occurs depending on disturbances and resource availability (Holway et al. Citation2002). It does not reproduce through nuptial flights; instead, queens mate with related males in their natal nests (Markin Citation1970). Local dispersal then occurs through budding when queens and workers walk from their parents’ nest to form new nests (Hölldobler & Wilson Citation1990; Heller et al. Citation2008). This behaviour explains its notorious capacity to form fast-growing intercontinental polydomous and polygynous super-colonies (Van Wilgenburg et al. Citation2010). The Argentine ant is active year-round during both night and day, with an omnivorous diet suited for a broad range of food resources (Vega and Rust Citation2001). It thrives on honeydew from trophobiotic hemipterans and is a highly aggressive competitor and predator, known for example to raid other ant nests in search of their food reserves and brood (Zee & Holway Citation2006; Shik & Silverman Citation2013).
In this paper, we report the first detection of the Argentine ant Linepithema humile on the island of La Réunion, a French overseas territory in the Indian Ocean. Specimens were morphologically identified and confirmed using a molecular marker (mitochondrial COI). We discuss potential implications for the local biota.
Materials and methods
Experimental sites
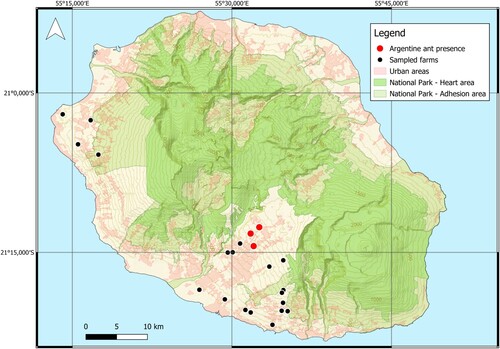
La Réunion is a subtropical and volcanic island in the Mascarene archipelago, 679 km to the east of Madagascar. Terrestrial land of La Réunion covers 2512 km² with a peak altitude at 3070 m. It hosts multiple microclimates due to this altitudinal gradient and particular topography, with a strong asymmetry in rainfall between windward (north and east) and leeward (south and west) zones (Morel et al. Citation2014; Davidson et al. Citation2023). As part of a larger project assessing biodiversity in market gardening crops of La Réunion, we sampled 22 fields from producers mainly located in the southern part of the island where most vegetable production is concentrated (). These fields encompassed a broad range of practices and crops, as well as surface areas (∼100 m² to over 3500 m²) and environmental conditions. Fields were at an altitude of 71 m to 1362 m and received an average annual rainfall of 1000 mm to 3000 mm. Two sampling periods were conducted, one in September–October 2022 corresponding to the end of the dry, cold season, and one in March 2023 which represents the peak of the hot and rainy season. Climatic data was collected and compiled from a dedicated CIRAD platform (https://smartis.re/METEOR, accessed 29.IX.2023).
Insect collection
In each field, two unidirectional cornet traps (Sarthou Citation2009) were placed head to tail on one of the borders, with collecting vessels containing 150 ml of 95% ethanol and left for 14 days on site. Also, three pitfall traps of 500 ml were buried, evenly spaced in a diagonal line throughout each field and left for seven days. These were filled with a 50/50 mixture of ethylene glycol and water for a total volume of 250 ml, with a drop of neutral soap and 2.5 g of sea salt added to the mixture to reduce water surface tension and increase tissue conservation time.
Molecular identification
Mixed insect samples from the cornet traps were stored in ethanol (95%) and sent to the Centre for Biology and Management of Populations (CBGP, Montpellier, France) for molecular identification. The metabarcoding protocols used followed the non-destructive DNA extraction, the two-steps PCR approach, library construction and post sequencing data treatment described in Sow et al. (Citation2019) with an improved set of primers targeting a longer region (458 bp) of the standard mitochondrial barcode fragment (BF3/BR2 CCHGAYATRGCHTTYCCHCG / TCDGGRTGNCCRAARAAYCA – (Elbrecht et al. Citation2019)). The contigs generated were identified using the BOLD system database (v.4: https://www.boldsystems.org/).
Morphological identification
Ten workers from the three locations were examined with a ZEISS© Stemi 08 stereomicroscope and photographs taken with a DeltaPix© Invenio EIII camera and the associated DeltaPix© Insight software. Taxonomic identification was carried out first at the genus level following Fisher and Bolton (Citation2016) and at the species level following Wild (Citation2007). To achieved morphological identifications, we performed the following morphological measurements: head length (HL) and width (HW), scape length (SL), eye length (EL), the number of ommatidia (NO), the number of teeth (NT) and the number of denticles (ND). We calculated several indexes: the cephalic index (CI), defined as (HW/HL) × 100, the ocular index (OI), defined as (EL/HW) × 100 and the scape index (SI) define as (SL/HL). Voucher specimens were mounted, dried and deposited at CBGP, Montpellier, France, in the CIRAD collection (Centre de Biologie pour la Gestion des Population Citation2018) and in the PVBMT (Plant Populations and Bio-aggressors in Tropical Ecosystems Joint Research Unit) collection.
Results
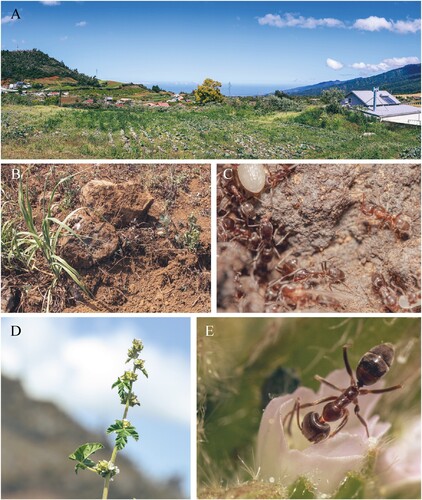
In three of the 22 sampled farms, we discovered the presence of an ant for which a metabarcoding analysis on a 458 base-pair COI fragment returned a 100% match with the Argentine ant Linepithema humile (GenBank accession number KX146468). After further analysis of the collected samples, we discovered a total of 215 individuals matching the morphology of this species. The first 148 discovered individuals were caught in September 2022 from a pitfall sample placed in a field (Farm 3) under organic management producing cabbage but bordering a field of Fabaceae plants heavily infested by aphids (). In the following months, 67 additional individuals were recovered from the same or two other farms nearby (less than 3 km away) under different management practices and crop systems (, ). All three farms were located in the southern central sampling area of the island, at altitudes ranging from 993 m to 1307 m. At the mean altitude of these occurrences (1148 m), the average low temperature for 2021 and 2022 was 11.6°C and the average high temperature was 22.5°C. Mean cumulated rainfall per year for the area during the same period was 1741 mm, with most rain during the hottest months, between December and April. In these invaded areas, land occupation is dominated by residential surfaces and market-gardening agriculture (, ; ).
Table 1. Details of the farm localities where individuals of the Argentine ant were sampled.
Morphological identification
Base morphometric measurements (head length, head width, and scape length) are consistent with those reported by Wild (Citation2007), and the range in the indices (CI and SI) are comparable (). The worker ants in our study exhibit a monomorphic size, characterized by a uniformly reddish-brown body and appendages (). Notably, the head is longer than it is broad (). The antennae consist of 12 segments, with the scape generally surpassing the length of the head and extending beyond its posterior margin. The eyes of these ants are relatively large and composed of numerous ommatidia. Furthermore, our observations reveal that the maxillary palp comprises six segments, while the labial palp consists of four segments. The mandibles are noteworthy for their numerous teeth and denticles. A distinctive feature of the genus Linepithema is the presence of an erect and easily identifiable scale on the petiole. Additionally, the dorsal side of the head, as well as the mesosome and abdominal tergites 2, 3 and 4 (including the petiole and the first two gastric segments), are devoid of setae. Conversely, the abdominal tergites and gastric tergites 5 and 6 (gastric segments 3 and 4) wear a pair of long setae. The mesotibia and the metatibia have one spur, the metatibial spur being pectinate.
Figure 3. Linepithema humile worker, individual collected in March 2023 from the municipality of Le Tampon (97430). A, Full-face view; B, lateral view; C, dorsal view.

Table 2. Morphological measurements and indices from 10 worker individuals.
Discussion
To our knowledge, this is the first molecular and morphological record of the Argentine ant Linepithema humile from La Réunion, and to a broader extent from the whole Malagasy region (encompassing Madagascar, the Seychelles, Comoros and the Mascarene islands). In parallel to our sampling, individuals of this species were independently collected by L. Colindre in August 2023 (Colindre Citation2023). The slight differences in the ranges of morphological measurement values could come from the lower number of specimens measured here (n = 10) than in Wild (Citation2007, n = 81). Although our sampling effort was not directed specifically towards this species, it was caught in three market-gardening farms from the same municipality (Le Tampon, postal code 97430) out of the 22 sampled across a larger area of the island. The establishment of the Argentine ant in that area is further confirmed by the observation from L. Colindre as the nest he discovered was located less than 1 km from our sampling site Farm 5 (21°12ʹ57.6ʺS 55°33ʹ00.0ʺE). Yearly temperature amplitude of the sites where it occurred are in agreement with reports and predictions of its preferences (Hartley et al. Citation2006; Wetterer et al. Citation2009). In addition, the area is dominated by fragmented urban and agricultural land, highly disturbed areas that are particularly profitable to invasive ants (Suarez et al. Citation1998; Ness & Bronstein Citation2004). The Argentine ant shows a preference for mesic habitats with high primary production, characteristics both found in the many agricultural fields of the area (Holway et al. Citation2002). In fact, abiotic conditions on the majority of the higher grounds of the island are projected to be suitable for future expansions of the invader (Hartley et al. Citation2006). Because this species was not detected in targeted Hymenoptera samplings up to the year 2006 (Blard Citation2006; Parnaudeau & Madl Citation2009), their establishment on the island is probably after this date, thus very recent as of writing. As for precedent invasions, we can hypothesize that the presence of this alien species is linked to maritime transport, as close to 500 ships arrive each year in the main port of La Réunion. The major commercial routes include mainland France, South Africa and Australia (https://reunion.port.fr/fr/accueil/), where the Argentine ant is already well established (Suarez et al. Citation2001; Wetterer et al. Citation2009).
As a young (2.1 Myr) and isolated volcanic island, La Réunion shows a rather poor myrmecofauna at around 52 species (AntWeb Citation2023; Supplementary Table 1), with no strict endemicity, although Camponotus aurosus Roger, 1863 is endemic to the Mascarene archipelago. The discovery of the Argentine ant adds the presence on this territory of a fourth invasive ant species out of the six considered to be the most damaging worldwide (Holway et al. Citation2002). The three previous notorious invaders are the yellow crazy ant, Anoplolepis gracilipes (Smith, 1857), the big-headed ant, Pheidole megacephala (Fabricius, 1793), and the tropical fire ant Solenopsis geminata (Fabricius, 1804). Next to the ants, a total of 34 species (both fauna and flora) from the 100 most invasive list are already present on La Réunion (MNHN Citation2023). Although subject to multiple biological invasions, La Réunion boasts the highest proportion of remaining native habitats of all the Mascarene islands. Around one-third of its land surface is considered to be intact and protected following inclusion in its national park in 2007, covering 42% of the territory (Strasberg et al. Citation2005; Fenouillas et al. Citation2021). This park is home to an important biodiversity, including many of the 900 vegetal species considered native to the island with 250 strictly endemic (CBNM Citation2023). In 2019, 3369 terrestrial arthropod species were listed, for which 31% (1044 species) were considered endemic to the island and an estimated 62% still remaining to be discovered (Legros et al. Citation2020). Due to this rich fauna and flora established in its unique landscape, La Réunion was granted the status of UNESCO World Heritage Site in 2010, highlighting the importance of conserving its diversity.
In this regard, although tramp species thrive particularly well in disturbed environments, they are also a menacing threat to natural environments (Vega & Rust Citation2001; Holway et al. Citation2002; Naughton et al. Citation2020). This is particularly true when invasion occurs on less resilient island ecosystems where interaction networks are often less complex and thus more prone to disturbance (Liebherr & Krushelnycky Citation2007; Bellard et al. Citation2017). The history of the invasion of Easter Island (F. Jacq, pers. comm.) or Hawaii by the Argentine ant presents good case-studies to predict potential establishment and consequences on La Réunion. Natural areas of Hawaii, even at higher altitudes, are indeed occupied and dominated by this invader, with important consequences (Cole et al. Citation1992; Krushelnycky et al. Citation2005; Hartley et al. Citation2010). Both La Réunion and Hawaii share similar ecological (multiple invasive species) and environmental characteristics (volcanic islands with strong abiotic gradients), with climate change increasing the suitable conditions at higher elevations where many endemic species occur.
As for the consequences of their invasion, the Argentine ant has for example proven to significantly alter invertebrate but also vertebrate composition upon invasion (Suarez et al. Citation2000; Rowles & O’dowd Citation2009). In particular, displacement and exclusion of native ant species is well documented, leading to subsequent biodiversity losses through cascading effects (Holway et al. Citation2002; Silverman & Brightwell Citation2008; Naughton et al. Citation2020). Vegetal composition is also susceptible to be affected by the arrival of the Argentine ant through several mechanisms. First, seed production is likely to decrease owing to interference (aggression) and competition (nectar resource) with pollinators (). Second, seed predation rates and seed dispersal are impacted following the exclusion of myrmecochorous species, changing floral population dynamics (Quilichini & Debussche Citation2000; Christian Citation2001; Witt et al. Citation2004).
Figure 2. A, View of Farm 5 where the first Argentine ant individuals were caught in October 2022. B, Large rocks around crop fields are typical nesting shelter sites of Linepithema humile. C, Disturbed ant individuals displacing brood. D, Malva parviflora L. (Malvales: Malvaceae) is a common plant in disturbed areas of La Réunion which hosted ant individuals on this farm. E, Ant individual feeding on floral nectar from M. parviflora.
In addition to their effects on natural areas, other particularly damaging consequences of the presence of this alien species concern crop production. The well-studied mutualism of this species with trophobiotic hemipterans has been proven to increase pest pressure on crops. They displace and foster pest hemipterans on the crops, and protect them from predation by parasitoids and predators, impeding natural biocontrol and increasing abundances of these agricultural pests (Vega & Rust Citation2001; Ness & Bronstein Citation2004). Aside from its negative effect, it is to be noted that L. humile could also present some benefits in crop production due to its predatory behaviour and ecological competitivity (Kamiyama et al. Citation2021; Schulze-Sylvester et al. Citation2022)
Means of control of the Argentine ant have long depended on chemical insecticides (for example fipronil and bifenthrin) (Rust et al. Citation2003; Klotz et al. Citation2007), although restrictions linked to sanitary hazards have generated the need for alternative solutions. However, no method (whether chemical or biological) has yet proven efficient to reduce Argentine ant populations in the field (Silverman & Brightwell Citation2008; Suiter et al. Citation2021). In crop production, the provision of carbohydrate solutions to ants is an approved method to disrupt mutualistic association with hemipterans, and thus reduce their impact (Pérez-Rodríguez et al. Citation2021; Correa et al. Citation2023). This could be tested with the Argentine ant, but caution should be taken as carbohydrate supply has sometimes led to facilitation of their invasive capacities in natural areas (Rowles & Silverman Citation2009).
Biotic interactions with already settled invasive ant species could present a barrier to their expansion on the island (LeBrun et al. Citation2007). An example is illustrated by the apparent reduction in distribution and population density of the yellow crazy ant, Anoplolepis gracilipes, which was one of the first invaders on the island (reported from 1895) and noted by Auguste Forel as having “infested the whole island and destroyed local fauna” (Forel Citation1895). To date, after the invasion of species such as the big-headed ant, Pheidole megacephala, or the tropical fire ant Solenopsis geminata, the yellow crazy ant is limited to anthropized areas and presents low population densities, probably due in part to intraguild competition (Blard Citation2006).
In summary, it is vital to firstly evaluate and monitor the present and future spread of the Argentine ant on the island of La Réunion, particularly regarding their presence in the national park area and in crop production systems. This could be done through field prospection and use of bait traps (Ujiyama & Tsuji Citation2018). Second, their impact on local fauna and flora should be assessed to decide of management strategies in these areas. Third, the trade-off between their potential services (pest control) and disservices (pest facilitation) should be evaluated in different production contexts. Lastly, means of control such as nest destruction or carbohydrate supply as well as competition with other species should be evaluated to help mitigate their negative impact.
Supplementary Table 1. Species list of known ants from the island of La Réunion as of October 2023.
Download MS Word (25.5 KB)Acknowledgements
We thank all producers involved for letting us do fieldwork on their fields. Thanks to Theo Delauney for his help during fieldwork and sorting of samples, and to Benoit Penel and Laure Benoit for assistance during the molecular identification process. We are grateful to the two anonymous reviewers who contributed to improving this manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplemental data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/00379271.2024.2311163.
Additional information
Funding
References
- AntWeb. 2023. AntWeb. Version 8.101. [Internet]. [accessed 20.XI.2023]. https://www.antweb.org.
- Bellard C, Cassey P, Blackburn TM. 2016. Alien species as a driver of recent extinctions. Biology Letters. 12(2):20150623. doi:10.1098/rsbl.2015.0623.
- Bellard C, Rysman J-F, Leroy B, Claud C, Mace GM. 2017. A global picture of biological invasion threat on islands. Nature Ecology & Evolution. 1(12):1862–1869. doi:10.1038/s41559-017-0365-6.
- Blard F. 2006. Les fourmis envahissantes de l’ile de La Réunion : interactions compétitives et facteurs d’invasion. La Réunion: Université de La Réunion.
- CBNM. 2023. Conservatoire Botanique National de Mascarin [Internet]. [accessed 13.XI.2023]. https://mascarine.cbnm.org/index.php/flore/statistiques/statistiques-sur-la-flore.
- Centre de Biologie pour la Gestion des Population. 2018. CBGP - Continental Arthropod Collection [Internet]. doi:10.15454/D6XAKL.
- Christian CE. 2001. Consequences of a biological invasion reveal the importance of mutualism for plant communities. Nature. 413(6856):635–639. doi:10.1038/35098093.
- Cole FR, Medeiros AC, Loope LL, Zuehlke WW. 1992. Effects of the Argentine ant on arthropod fauna of Hawaiian high-elevation shrubland. Ecology. 73(4):1313–1322. doi:10.2307/1940678.
- Colindre L. 2023. Découverte de Linepithema humile sur l’île de la Réunion & nouvelle mention pour l’espèce Cyphomyrmex minutus Mayr, 1862 [Internet]. [accessed 4.I.2024]. https://doi.org/10.5281/ZENODO.10223027.
- Correa P, Wäckers F, Brévault T, Bouvery F, Detrain C, Chailleux A. 2023. Sugar feeders reduce weaver ants’ drawbacks when used as biological control agents in mango orchards. Biological Control. 177:105103. doi:10.1016/j.biocontrol.2022.105103.
- Davidson AS, Malet-Damour B, Praene JP. 2023. A new microclimate zoning method based on multivariate statistics: The case of Reunion Island. Urban Climate. 52:101687. doi:10.1016/j.uclim.2023.101687.
- Diagne C, Leroy B, Vaissière A-C, Gozlan RE, Roiz D, Jarić I, Salles J-M, Bradshaw CJA, Courchamp F. 2021. High and rising economic costs of biological invasions worldwide. Nature. 592(7855):571–576. doi:10.1038/s41586-021-03405-6.
- Elbrecht V, Braukmann TWA, Ivanova NV, Prosser SWJ, Hajibabaei M, Wright M, Zakharov EV, Hebert PDN, Steinke D. 2019. Validation of COI metabarcoding primers for terrestrial arthropods. PeerJ. 7:e7745. doi:10.7717/peerj.7745.
- Fenouillas P, Ah-Peng C, Amy E, Bracco I, Dafreville S, Gosset M, Ingrassia F, Lavergne C, Lequette B, Notter J-C, et al. 2021. Quantifying invasion degree by alien plants species in Réunion Island. Austral Ecology. 46(7):1025–1037. doi:10.1111/aec.13048.
- Fisher BL, Bolton B. 2016. Ants of Africa and Madagascar: a guide to the genera. Oakland, CA: Univ of California Press.
- Forel A. 1895. Nouvelles fourmis de diverses provenances, surtout d'Australie. Annales de la Société Entomologique de Belgique. 39: 41–49.
- Global Invasive Species Database (GISD). 2023. Species profile Linepithema humile [Internet]. [accessed 22.IX.2023]. http://www.iucngisd.org/gisd/species.php?sc=127.
- Gómez C, Abril S. 2008. Linepithema humile (Argentine ant). CABI Compendium. CABI Compendium:30839. https://doi.org/10.1079/cabicompendium.30839.
- Guénard B, Weiser MD, Gómez K, Narula N, Economo EP. 2017. The Global Ant Biodiversity Informatics (GABI) database: synthesizing data on the geographic distribution of ant species (Hymenoptera: Formicidae) [Internet]. [accessed 22.IX.2023]. doi:10.25849/MYRMECOL.NEWS_024:083.
- Hartley S, Harris R, Lester PJ. 2006. Quantifying uncertainty in the potential distribution of an invasive species: climate and the Argentine ant. Ecology Letters. 9(9):1068–1079. doi:10.1111/j.1461-0248.2006.00954.x.
- Hartley S, Krushelnycky PD, Lester PJ. 2010. Integrating physiology, population dynamics and climate to make multi-scale predictions for the spread of an invasive insect: the Argentine ant at Haleakala National Park, Hawaii. Ecography. 33(1):83–94. doi:10.1111/j.1600-0587.2009.06037.x.
- Heller NE, Ingram KK, Gordon DM. 2008. Nest connectivity and colony structure in unicolonial Argentine ants. Insectes Sociaux. 55(4):397–403. doi:10.1007/s00040-008-1019-0.
- Hölldobler B, Wilson EO. 1990. The Ants. Cambridge, UK: Springer-Verlag Berlin Heidelberg, Harvard University Press.
- Holway DA, Lach L, Suarez AV, Tsutsui ND, Case TJ. 2002. The causes and consequences of ant invasions. Annual Review of Ecology and Systematics. 33(1):181–233. doi:10.1146/annurev.ecolsys.33.010802.150444.
- Kamiyama MT, Matsuura K, Yoshimura T, Yang C-CS. 2021. Predation of the brown marmorated stink bug, Halyomorpha halys by the Japanese acrobat ants, Crematogaster matsumurai and Crematogaster osakensis. Biological Control. 157:104570. doi:10.1016/j.biocontrol.2021.104570.
- Klotz JH, Rust MK, Greenberg L. 2007. An evaluation of several urban pest management strategies to control argentine ants (Hymenoptera: Formicidae). Sociobiology 50(2):391–98.
- Krushelnycky PD, Loope LL, Reimer NJ. 2005. The ecology, policy, and management of Ants in Hawaii [Internet]. [accessed 13.XI.2023]. http://hdl.handle.net/10125/103.
- Kumschick S, Gaertner M, Vilà M, Essl F, Jeschke JM, Pyšek P, Ricciardi A, Bacher S, Blackburn TM, Dick JTA, et al. 2015. Ecological impacts of alien species: quantification, scope, caveats, and recommendations. BioScience. 65(1):55–63. doi:10.1093/biosci/biu193.
- LeBrun EG, Tillberg CV, Suarez AV, Folgarait PJ, Smith CR, Holway DA. 2007. An experimental study of competition between fire ants and Argentine ants in their native range. Ecology. 88(1):63–75. doi:10.1890/0012-9658(2007)88[63:AESOCB]2.0.CO;2.
- Legros V, Rochat J, Reynaud B, Strasberg D. 2020. Known and unknown terrestrial arthropod fauna of La Réunion Island, Indian Ocean. Journal of Insect Conservation. 24(1):199–217. doi:10.1007/s10841-019-00188-0.
- Liebherr JK, Krushelnycky PD. 2007. Unfortunate encounters? Novel interactions of native Mecyclothorax, alien Trechus obtusus (Coleoptera: Carabidae), and Argentine ant (Linepithema humile, Hymenoptera: Formicidae) across a Hawaiian landscape. In: New TR, editor. Beetle Conservation [Internet]. Dordrecht: Springer Netherlands; p. 61–73; [accessed 22.IX.2023]. doi:10.1007/978-1-4020-6047-2_8.3.
- Lowe S, Browne M, Boudjelas S, De Poorter M. 2000. 100 of the world’s worst invasive alien species: a selection from the global invasive species database. New Zealand: Invasive Species Specialist Group Auckland.
- Markin GP. 1970. The seasonal life cycle of the Argentine Ant, Iridomyrmex humilis (Hymenoptera: Formicidae), in Southern California. Annals of the Entomological Society of America. 63(5):1238–1242. doi:10.1093/aesa/63.5.1238.
- Mehta SV, Haight RG, Homans FR, Polasky S, Venette RC. 2007. Optimal detection and control strategies for invasive species management. Ecological Economics. 61(2):237–245. doi:10.1016/j.ecolecon.2006.10.024.
- MNHN. 2023. Compteur biodiversité d’outre-mer. Compteur de biodiversite Outre-mer [Internet]. [accessed 13.XI.2023]. https://biodiversite-outre-mer.fr/.
- Morel B, Pohl B, Richard Y, Bois B, Bessafi M. 2014. Regionalizing rainfall at very high resolution over La Réunion island using a regional climate model. Monthly Weather Review. 142(8):2665–2686. doi:10.1175/MWR-D-14-00009.1.
- Naughton I, Boser C, Tsutsui ND, Holway DA. 2020. Direct evidence of native ant displacement by the Argentine ant in island ecosystems. Biological Invasions. 22(2):681–691. doi:10.1007/s10530-019-02121-7.
- Ness JH, Bronstein JL. 2004. The effects of invasive ants on prospective ant mutualists. Biological Invasions. 6(4):445–461. doi:10.1023/B:BINV.0000041556.88920.dd.
- Ogden NH, Wilson JRU, Richardson DM, Hui C, Davies SJ, Kumschick S, Le Roux JJ, Measey J, Saul W-C, Pulliam JRC. 2019. Emerging infectious diseases and biological invasions: a call for a One Health collaboration in science and management. Royal Society Open Science. 6(3):181577. doi:10.1098/rsos.181577.
- Parnaudeau R, Madl M. 2009. Liste des Hyménoptères des îlots coralliens français et mauriciens de l’océan Indien occidental. Bulletin de la Société entomologique de France. 114(4):453–462. doi:10.3406/bsef.2009.2718.
- Pérez-Rodríguez J, Pekas A, Tena A, Wäckers FL. 2021. Sugar provisioning for ants enhances biological control of mealybugs in citrus. Biological Control. 157:104573. doi:10.1016/j.biocontrol.2021.104573.
- Quilichini A, Debussche M. 2000. Seed dispersal and germination patterns in a rare Mediterranean island endemic (Anchusa crispa Viv., Boraginaceae). Acta Oecologica. 21(6):303–313. doi:10.1016/S1146-609X(00)01089-4.
- Rowles AD, O’dowd DJ. 2009. Impacts of the invasive Argentine ant on native ants and other invertebrates in coastal scrub in south-eastern Australia. Austral Ecology. 34(3):239–248. doi:10.1111/j.1442-9993.2008.01922.x.
- Rowles AD, Silverman J. 2009. Carbohydrate supply limits invasion of natural communities by Argentine ants. Oecologia. 161(1):161–171. doi:10.1007/s00442-009-1368-z.
- Rust MK, Reierson DA, Klotz JH. 2003. Pest management of argentine ants (Hymenoptera: Formicidae). Journal of Entomological Science. 38(2):159–169. doi:10.18474/0749-8004-38.2.159.
- Sarthou J-P. 2009. Le piège cornet unidirectionnel, nouveau piège entomologique d’interception. L’Entomologiste. 65(2):107–108.
- Schulze-Sylvester M, Sylvester F, Torres VM, Paris CI, Corronca JA. 2022. Alien vs. herbivore: ant-mediated plant defense as an option for biological control of leafcutter ants. Agronomy for Sustainable Development. 42(5):104. doi:10.1007/s13593-022-00826-z.
- Shik JZ, Silverman J. 2013. Towards a nutritional ecology of invasive establishment: aphid mutualists provide better fuel for incipient Argentine ant colonies than insect prey. Biological Invasions. 15(4):829–836. doi:10.1007/s10530-012-0330-x.
- Silverman J, Brightwell RJ. 2008. The Argentine ant: challenges in managing an invasive unicolonial pest. Annual Review of Entomology. 53(1):231–252. doi:10.1146/annurev.ento.53.103106.093450.
- Sow A, Brévault T, Benoit L, Chapuis MP, Galan M, Coeur d’acier A, Delvare G, Sembène M, Haran J. 2019. Deciphering host-parasitoid interactions and parasitism rates of crop pests using DNA metabarcoding. Scientific Reports. 9(1):1–12. doi:10.1038/s41598-019-40243-z.
- Strasberg D, Rouget M, Richardson DM, Baret S, Dupont J, Cowling RM. 2005. An assessment of habitat diversity and transformation on La Réunion Island (Mascarene Islands, Indian Ocean) as a basis for identifying broad-scale conservation priorities. Biodiversity & Conservation. 14(12):3015–3032. doi:10.1007/s10531-004-0258-2.
- Suarez AV, Bolger DT, Case TJ. 1998. Effects of fragmentation and invasion on native ant communities in coastal southern California. Ecology. 79(6):2041–2056. doi:10.1890/0012-9658(1998)079[2041:EOFAIO]2.0.CO;2.
- Suarez AV, Holway DA, Case TJ. 2001. Patterns of spread in biological invasions dominated by long-distance jump dispersal: insights from Argentine ants. Proceedings of the National Academy of Sciences. 98(3):1095–1100. doi:10.1073/pnas.98.3.1095.
- Suarez AV, Richmond JQ, Case TJ. 2000. Prey selection in horned lizards following the invasion of Argentine Ants in southern California. Ecological Applications. 10(3):711–725. doi:10.1890/1051-0761(2000)010[0711:PSIHLF]2.0.CO;2.
- Suiter DR, Gochnour BM, Holloway JB, Vail KM. 2021. Alternative methods of ant (Hymenoptera: Formicidae) control with emphasis on the Argentine ant, Linepithema humile. Insects. 12(6):487. doi:10.3390/insects12060487.
- Tsutsui ND, Suarez AV. 2003. The colony structure and population biology of invasive ants. Conservation Biology. 17(1):48–58. doi:10.1046/j.1523-1739.2003.02018.x.
- Ujiyama S, Tsuji K. 2018. Controlling invasive ant species: a theoretical strategy for efficient monitoring in the early stage of invasion. Scientific Reports. 8(1):8033. doi:10.1038/s41598-018-26406-4.
- Van Wilgenburg E, Torres CW, Tsutsui ND. 2010. The global expansion of a single ant supercolony. Evolutionary Applications. 3(2):136–143. doi:10.1111/j.1752-4571.2009.00114.x.
- Vega SJ, Rust MK. 2001. The Argentine ant - a significant invasive species in agricultural, urban and natural environment. Sociobiology. 37:3–25.
- Wetterer JK, Wild AL, Suarez AV, Roura-Pascual N, Espadaler X. 2009. Worldwide spread of the Argentine ant, Linepithema humile (Hymenoptera: Formicidae). Myrmecological News. 12:187–194.
- Wild AL. 2007. Taxonomic revision of the ant genus Linepithema (Hymenoptera: Formicidae). California: Univ of California Press.
- Witt ABR, Geertsema H, Giliomee JH. 2004. The impact of an invasive ant, Linepithema humile (Mayr) (Hymenoptera : Formicidae), on the dispersal of the elaiosome-bearing seeds of six plant species. African Entomology. 12(2):223–230. doi:10.10520/EJC32602.
- Zee J, Holway D. 2006. Nest raiding by the invasive Argentine ant on colonies of the harvester ant, Pogonomyrmex subnitidus. Insectes Sociaux. 53(2):161–167. doi:10.1007/s00040-005-0853-6.