Abstract
Research on the effects of selenium nanoparticles (Se-NPs), particularly in Japanese quails, is lacking, especially regarding the potential for DNA damage. Therefore, this study aimed to investigate the impact of administering 0.2 and 0.4 mg/kg of Se-NPs on the growth performance, DNA integrity, and histopathological alterations of the liver, lung, kidney, and heart in quails. A total of 480 one-day-old Japanese quails were divided into three experimental groups as follows: Group 1 served as the control and received only basic feed, while Group 2 and 3 received 0.2 mg/kg and 0.4 mg/kg of Se-NPs via oral gavage. Our results suggested that, birds fed with Se-NPs at both levels significantly (p < .01) reduced feed intake, however, weight gain was significantly (p < .01) increased in quails supplemented with 0.2 mg/kg. Similarly, feed conversion ratio (FCR) was significantly (p < .01) reduced in group supplemented with 0.2 mg/kg Se-NPs. White blood cells increased significantly (P0.01) in 0.4 mg/kg while haemoglobin and red cell distribution width decreased (p < .01) in the same group. Both treatment regimens resulted in DNA damage and histopathological alterations; however, the adverse effects were more prominent in the group receiving the higher dose of 0.4 mg/kg. These findings indicate that the lower dose of 0.2 mg/kg may have beneficial effects on growth. However, the higher dose of 0.4 mg/kg not only negatively impacts growth but also leads to histopathological alterations in major organs of the body and DNA damage as well.
Introduction
The field of nanotechnology is undergoing rapid expansion and encompasses diverse realms of academic research (Aziz et al. Citation2022). Its potential for making substantial strides in human and animal health is noteworthy, especially in domains such as bolstering resistance against pathogens, employing antioxidants, degrading toxins, and enhancing nutrient efficiency (Reda et al. Citation2020, Citation2021). Selenium, found in organic (seleno-cysteine and seleno-methionine) and inorganic (selenate and selenite) forms, is relatively rare in nature. Human activities, such as refining, fossil fuel combustion, agricultural practices, oil smelting, and industrial mine sewage discharge, have led to significant contamination issues in soils and water bodies (Khan et al. Citation2022; Hassan et al. Citation2023). This element bioaccumulates in polluted areas, entering plants, then livestock that consumes these plants, and ultimately reaching humans who consume the animals. Selenium is crucial for various functions in the human body, including metabolism, thyroid hormone regulation, DNA synthesis, reproduction, and protection against oxidative and infectious damage, being a component of seleno-proteins and seleno compounds (Kumar and Prasad Citation2021; Ostovar et al. Citation2022).
Poultry feed additives currently encompass inorganic selenium forms like sodium selenite, and organic forms such as selenomethionine, selenocysteine, and Se-enriched yeast. The supplementation of selenium has been shown to enhance the growth performance, antioxidant status and immune system of heat-stressed broiler chickens (Bami et al. Citation2022). Selenium, a crucial trace element in the diet, plays a vital role in both internal and external organ function, contributing to animal growth, immunity, hormonal balance, and fertility (Kassim et al. Citation2022). Selenium nanoparticles have demonstrated significantly higher efficiency compared to selenium salts, and they are also less toxic (Chen et al. Citation2022).
Selenium nanoparticles (Se-NPs) have been successfully synthesized through various green nanotechnology approaches, including the use of plant extracts, as reported by Zambonino et al. (Citation2021) and Medina Cruz et al. (Citation2018). Green synthesis offers an eco-friendly method for nanoparticle synthesis, utilizing both unicellular and multicellular living organisms, such as yeast, fungi, bacteria, plant tissues, and algae, as natural reducing and stabilizing agents (Malyugina et al. Citation2021). Multiple studies have demonstrated that the form and dosage of selenium in the diet can significantly impact the growth performance, antioxidative properties, immunity and meat quality attributes in broilers. Nevertheless, as per the authors’ knowledge, there is a scarcity of information regarding the utilization of nano-Se in Japanese quails diets. Therefore, the main objective of the present study was to evaluate the impact of different levels of biologically synthesized nanoselenium supplementation at the rate of 0.2 and 0.4 mg/kg on the growth performance, hematological parameters, histopathological changes in vital organs, and DNA damage in Japanese quails.
Materials and methods
Extraction of capsicum extract
The contaminants associated with Capsicum annum were meticulously removed by washing them with double-distilled water seven times. The cleaned components were then sliced into small pieces and allowed to air-dry in the shade for a period of 15 d. Subsequently, the dried pieces were ground into a powder using a grinder. The resulting 500 g powder of Capsicum annum was subjected to a maceration process, wherein it was added to 1000 ml of ethanol. The mixture underwent filtration using filter paper to separate the filtrate. The obtained filtrate was processed in a rotary evaporator for approximately 15 min, and the resulting extract was further treated in a vacuum evaporator until a constant weight was achieved. This process yielded a dry cake extract.
Synthesis of selenium nanoparticles (Se-NPs)
For the synthesis of selenium nanoparticles (Se-NPs), 50 ml of the Capsicum annum extract was mixed with 0.263 g of selenious acid (H2SeO3). Over a 15-h period, the pH of the mixture was maintained at 5.4 by adding NaOH (sodium hydroxide), resulting in a red color appearance (NaOH was added to adjust the pH from 4.8). The mixture was then centrifuged at 12,000 rpm for 4 min at room temperature. The deposited material was washed with deionized water and left in tubes for complete drying before being weighed (Batra et al. Citation2017).
Birds, experimental design and management
A total of 480 one-day-old C. coturnix japonica chicks were randomly allocated into three groups, each consisting of eight replicates. Group 1 served as the control group, receiving a normal basal diet. Groups 2 and 3 were supplemented with 0.2 and 0.4 mg/kg respectively. The quails were housed in wire cages under consistent housing and management conditions, adhering to standard hygiene protocols for a 10-day acclimatization period. The temperature was maintained at approximately 36 °C, and humidity ranged from 50 to 70%. Throughout the trial, the birds were provided with commercial broiler feed (). They had unrestricted access to water and food, and their light cycle consisted of 18 h of sunlight and 6 h of darkness. All groups followed identical lighting, feeding, and watering procedures throughout the experiment.
Table 1. Feed ingredients and nutrient content of the basal diets.
Growth performance
The body weight gain (BWG) and feed intake of the birds for the experimental period (1–35 d) was calculated on weekly basis. The obtained data was used to calculate FCR. Mortality was recorded as occurred (Foroutankhah et al. Citation2019).
Haemotological analysis
At the 35th day of the study, blood samples were obtained from 3 chicks per replicate through cardiac puncture using EDTA tubes. The collected blood samples with EDTA were carefully placed into properly labeled and sterilized tubes, specifically designated for the analysis of hematological parameters. Hematological parameters include lymphocytes, white blood cells, mid-range, red blood cells, granulocytes, haemoglobin, mean corpuscular volume, haematocrit, platelet count, mean corpuscular haemoglobin and mean corpuscular hemoglobin concentration.
Histopathological examination
At the conclusion of the study, three birds per replicate were slaughtered, and their skin was removed. Liver, heart, and kidneys were then aseptically extracted. These tissues were preserved in a 10% formalin neutral solution for subsequent evaluation of potential histopathological alterations. Following preservation, the tissues were processed and sliced to approximately 4 μm thickness. Finally, the tissues were stained with hematoxylin-eosin according to the method outlined by Landy et al. (Citation2020).
Comet assay
DNA damage in japanese quails was assessed using the Comet assay following the protocol outlined by Singh et al. (Citation1988) with slight modifications. Ten samples from each group were analyzed using the Comet assay technique.
Statistical analysis
The data were processed using Microsoft Excel (USA) and Statistica version 12.0 (CZ). Statistical analysis was performed using a one-way analysis of variance (ANOVA) with sex as a covariate can be used to adjust for any potential differences between male and female quails. Scheffe’s test was then applied to determine significant differences with confidence. A significance level of p < .05 was used to identify statistically meaningful distinctions between the groups.
Results
displays the weekly feed intake of C. coturnix japonica under various Se-NPs treatments. The findings indicated that at 0.4 mg/kg Se-NPs, the feed intake was significantly (p < .01) lower compared to both 0.2 mg/kg and the control group on weekly basis except week 1.
Table 2. Feed intake, weight gain and feed conversion ratio in Japanese quails supplemented with different levels of selenium nanoparticle.
presents the weekly body weight of C. coturnix japonica under different treatments of Se-NPs. Weight gain was significantly (p < .01) higher in quails, except in the first and second weeks, when supplemented with 0.2 mg/kg Se-NPs, compared to the 0.4 mg/kg and control groups.
displays the weekly Feed Conversion Ratio (FCR) of C. coturnix japonica under different treatments of Se-NPs. FCR was significantly (p < .01) lower in quails that received 0.2 mg/kg Se-NPs compared to the control and those supplemented with 0.4 mg/kg selenium nanoparticles. Linear and quadratic effects were recorded for feed intake, weight gain and FCR for different Se-NP compared to the control.
displays the cell Blood Count (CBC) of C. coturnix japonica reared under control and Se-NPs supplementation. The results indicated that WBC count was significantly (p < .01) higher in quails supplemented with 0.2 mg/kg selenium nanoparticles compared to 0.4 mg/kg and the control group. However, lymphocyte percentage decreased significantly (p < .01) in the treatment group compared to the control. Hemoglobin concentration decreased significantly (p < .01) in the 0.4 mg/kg supplemented selenium nanoparticles group. All other parameters did not change significantly between the control and the treatment groups. Linear and quadratic effects were seen for WBCs, lymphocytes and red cell distribution width in the treatment groups compared to the control.
Table 3. Complete blood count of Japanese quails supplemented with different levels of selenium nanoparticles.
showed various comet parameters observed in the blood of C. coturnix japonica in response to different doses of Se-NPs. The results indicate that L tail, L comet, tail DNA, TM, and OTM increased significantly (p < .001) in quails supplemented with 0.4 ppm selenium nanoparticles. However, head DNA decreased significantly (p < .001) in the higher dose (0.4 ppm) of selenium nanoparticles. The orthogonal results also showed linear and quadratic effects of the Se-NPs in comparison to the control.
Table 4. Comet parameters observed in blood of Japanese quails supplemented with different levels of selenium nanoparticles.
Histological analysis of liver
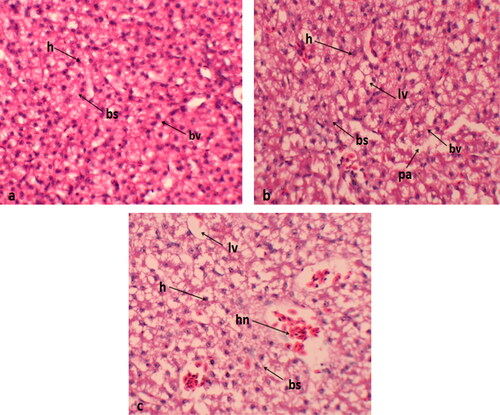
Histological sections of the liver from the control group displayed normal histological architecture. In contrast, the liver from birds supplemented with 0.4 mg/kg exhibited blood sinusoid dilation and mild hepatocellular cytoplasmic vacuolization ().
Figure 1. Histological sections of liver (a) normal histological features; (b) dilation of sinusoids and mild vacuolization of hepatocellular cytoplasm in 0.2 mg/kg group; (c) Quails received 0.4 mg/kg Se-NPs showing numerous necrotic hepatocytes, bs: blood sinusoid; h: hepatocyte; lv: lipid vacuole; bv: blood vessels; hn: hepatocyte necrosis.
Histological analysis of heart
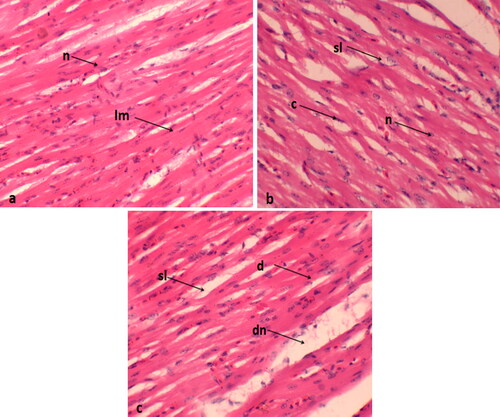
In the control group, myocardial fibers exhibited regular organization, normal nuclei, and a well-arranged pattern with distinct borders. However, the treatment group exposed to 0.4 mg/kg displayed muscle splitting and nuclei dislocation ().
Histological analysis of kidney
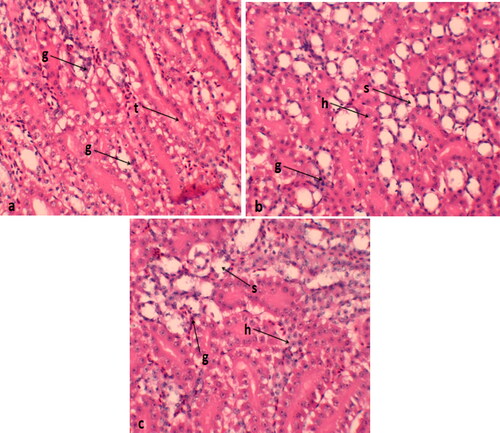
The control group exhibits normal kidney tissue, consisting of glomeruli and renal tubules bordered by simple cuboidal epithelial tissue. However, the majority of the epithelial cells lining the renal tubules in this group showed significant signs of degeneration and swelling. These histological alterations indicated variations in the size of the glomeruli, with the 0.4 mg/kg supplementation displaying more pronounced swelling compared to the 0. 2 mg/kg supplemented quails ().
Figure 3. Histological features of kidney of Japanese quails supplemented with selenium nanoparticles (a) control group; (b) Se-NP treatment at the rate of 0.2 mg/kg; (c) Se-NP treatment at the rate of 0.4 mg/kg. g: glomerular; t: renal tubules; s: swelling; h: hemorrhage; s: sloughing of epithelial cells.
DNA damage assay
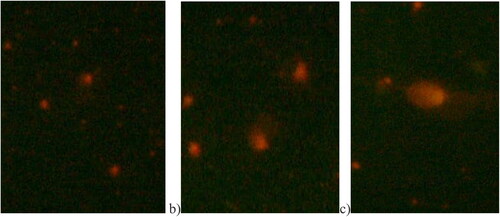
The results from the comet assay are illustrated in . In the control group, cells appear normal without a tail. In quails supplemented with 0.2 mg/kg, cells display a distinct Comet-like structure with both a tail and a head. Furthermore, in quails supplemented with 0.4 mg/kg, cells exhibit an even more pronounced Comet tail.
Figure 4. Comparison of DNA damage images of C. coturnix japonica exposed to Se-NPs doses (0.2 mg/kg and 0.4 mg/kg). After the comet assay process, pictures were taken at magnification ×30X. (a) Control, normal cell without a tail; (b) 0.2 mg/kg Se-NPs treated group cell showing a tail and a head like a comet; (c) 0.4 mg/kg Se-NPs treated group showing longer tail.
Discussion
Selenium plays a crucial role as a component of enzymes as a component of antioxidant enzymes to neutralize free radicals generated during oxidation processes, owing to its antioxidant properties. It is a part of unique active proteins called selenoproteins (Kieliszek and Błażejak Citation2013). Selenium nanoparticles, while having low toxicity, can be damaging even in minimal amounts. In comparison to other selenium species, Se-NPs exhibit potent anticancer efficacy with fewer associated toxicity issues (Ifijen et al. Citation2023). In the context of current growth performance, especially FCR, Se-NPs at low doses (0.2 mg/kg) have demonstrated a higher growth rate than the control and high-dose groups. Jiang et al. (Citation2009) emphasized selenium’s essential role as a trace element in various physiological processes. These nanoparticles offer enhanced bioavailability, facilitating improved absorption and utilization by quails. Selenium, a crucial component of selenoproteins, influences antioxidant defense mechanisms and supports immune function. It also plays a vital role in thyroid hormone metabolism, impacting overall growth and development. The use of selenium nanoparticles in quail diets has shown promising results in enhancing growth parameters, including weight gain and feed efficiency, highlighting their potential as a nutritional supplement for optimizing the growth performance of Japanese quails. Dawood et al. (Citation2020) found that the inclusion of Se-NPs significantly improved feed conversion ratio and feed intake. Similarly, Ahmadi et al. (Citation2018) demonstrated that diets containing Se-NPs at levels of 0.2 and 0.4 mg/kg improved the development and carcass characteristics of broilers without any negative effects on their internal organs. Eid et al. (Citation2023) illustrated how bird performance may be enhanced by selenium in nano form, attributing these benefits to antioxidant activity, encouraging increased protein production by the bird’s enzymatic system, and consequently improving all aspects of the physiological response.
Concerning the feed intake of quails, the results indicated a significant reduction in total feed intake when quails were fed with different levels of Se-NPs. These findings are consistent with the observations of Eldeeb and Ibrahim (Citation2023), who noted that various levels of Se-NPs at rates of 0.2, 0.4, and 0.6 ppm led to a decrease in feed intake in ducks. Similarly, El-Kazaz et al. (Citation2020) showed in an experiment of Japanese quails that administering 0.2 mg/kg Nano-Se significantly decreased feed intake. Similarly, Jamima et al. (Citation2020) recorded that a dose of 0.15 mg/kg Nano-Se in birds had the lowest feed consumption and improved FCR. Moreover, reduced feed intake in broilers in response to 0.3 mg/kg nano-selenium was also reported by Eid et al. (Citation2022).
The current study revealed a significantly lower FCR in quails supplemented with 0.2 mg/kg Se-NPs. These findings align with the observations of Eldeeb and Ibrahim (Citation2023), who reported improved FCR in ducks with various levels of selenium nanoparticles at rates of 0.2, 0.4, and 0.6 ppm. The advantageous effects of nano selenium on growth traits may be attributed to its inherent chemical as well as physical properties, which are biologically produced. Nanoparticles possess a greater surface area, which increases the available surface area, potentially enhancing mineral digestion, bioavailability, and utilization (Sa’aci et al. Citation2021). Our results are in agreement with Ahmadi et al. (Citation2018), who found improved FCR in broilers fed a diet enriched with nano-Se (0.3 mg/kg). Likewise, Saleh and Ebeid (Citation2019) discovered that in response to 0.5 mg nano-Se/kg feed enhanced FCR in broilers. Furthermore, Sa’aci et al. (Citation2021) demonstrated that broilers fed diets containing 0.10–0.25 mg of NS exhibited improved FCR. Additionally, Eid et al. (Citation2022) recorded that 0.3 mg/kg nano-selenium exhibited better FCR ccompared to those on the control diet, which had lower FCR values.
Nanoparticles provide an opportunity to provide enhanced bioavailability and therapeutic agents thorough high surface availability and size of the particles (Naz et al. Citation2024). Experiments have proved that nanoparticles showed better transport, uptake and absorption capacity (Aslam et al. Citation2023) leading to improved growth performance (Eldeeb and Ibrahim Citation2023). Different factors such as dose, duration, strain of birds and other experimental conditions may be responsible for the changes in the growth performance.
In terms of the hematological analysis of quails, fed different amounts of Nano-Se, a noteworthy increase was observed in blood lymphocyte and WBCs count a. These findings align with the results reported by Eldeeb and Ibrahim (Citation2023), who observed that different levels of selenium nanoparticles at rates of 0.2, 0.4, and 0.6 ppm increased total leukocyte counts and lymphocytes in ducks.
Leukocytosis was attributed to an inflammatory response in the intestinal tract and bone marrow hyperplasia (Fotouh et al. Citation2014). In the current study, granulocytes, MCH, MCV, MCHC and platelets did not change significantly between the control and the treatment groups. In the same vain, Eldeeb and Ibrahim (Citation2023) reported that platelets count, MCHC, MCH, MCV and Hct, did not change significantly between the control and the selenium nanoparticles treated ducks. These findings align with those reported by Boostani et al. (Citation2015), reported that in response to 0.3 mg/kg of Nano-Se had no significant impact on TEC, and PCV in broilers. Similarly, Mohamed et al. (Citation2016) demonstrated in Sinai chick that incorporating nanoselenium did not affect eosinophils and monocytes. In another study, Alagawany et al. (Citation2021) determined that supplementation different doses of Se-NP rangning from 0.2 to 0.6 g/kg had no influence on granulocytes, RBCs, hematocrit, and MCV in growing quail. Conversely, Jamima et al. (Citation2020) reported that a dose of 0.15 mg/kg Nano-Se significantly elevated levels of Hb, TEC, and PCV, while the effects on WBCs count was not affected significantly. Furthermore, Eid et al. (Citation2022) demonstrated that dose of Se-NPs at dose rate of 0.3 mg/kg in broiler diets had no significant impact on hematological parameters compared to the control group.
In the current study, histopathological examinations were employed to investigate the cytotoxic effects of Se-NPs on various tissues. The examinations revealed dose-dependent pathological alterations in all examined organs, including the lungs, heart, liver, and kidneys, in groups that received Se-NPs at either 0.2 or 0.4 mg per kilogram of body weight. The most noticeable lesions included cellular cytoplasmic vacuolization, dislocation of the nucleus, swelling, degeneration, necrosis, muscle splitting, hemorrhage, congestion, and a mild to moderate degree of inflammation. Similar results were observed by Akhtar et al. (Citation2016) and Khalaf et al. (Citation2017), who found that NPs induce inflammation, oxidative stress, and consequent damage to proteins, cellular and cytoplasmic organelle membranes, and DNA structures. The visible clinical damages in tissues might be connected to oxidative stress generated by NPs, as well as the associated increase in Se levels, which are considered hazardous to cells and tissues. Lin et al. (Citation2009) and Yang et al. (Citation2009) suggested that the primary mechanism underlying the toxicity of nanoparticles (NPs) is their ability to generate free radicals. Our research findings also support a significant adverse effect of Se-NPs on the major organs of Japanese quails, which could be linked the toxic effects of Se-NPs in our study and closely associated with the increasing levels of deposition of Se in these tissues. Previous research has concluded that NPs dissociate in the acidic environment of the stomach but remain unaffected in the neutral pH of the small intestine (Dang et al. Citation2021). Within the small intestine, NPs accumulate in the villi, disperse into the bloodstream, and subsequently distribute to various organs, thereby inducing cytotoxicities (De Jong et al. Citation2019).
Current pathological investigations into the genotoxicity of Se-NPs, validated by a comet assay, revealed higher DNA damage in the groups that received 0.4 ppm Se-NPs compared to the control group. Previous research has indicated that Se-NPs can impact cell viability, oxidative equilibrium, and DNA integrity (Karlsson Citation2010; Wang et al. Citation2011). Monteith and Skaar (Citation2021) demonstrated that selenium nanoparticles might interfere with DNA repair mechanisms, leading to the consequnces of DNA damage. This results in base lesions and breakage of DNA strands through reactions with both deoxyribose sugars and the nucleobases of DNA. Additionally, ROS oxidizes DNA, and Se hinders DNA repair and transcriptional regulation, posing a threat to the stability of genetic information. These findings align with other studies in chickens (Morsy et al. Citation2014), fish (Kousar and Javed Citation2015; Nikdehghan et al. Citation2018), and birds (Hussain et al. Citation2011; Auon et al. Citation2014).
Conclusion
The study revealed improved body weight gain and feed conversion ratio (FCR) in Japanese quails administered with 0.2 mg/kg of Se-NPs. However, a higher dose (0.4 mg/kg) led to reduced growth performance, accompanied by significant histological damage and genotoxic effects.
Authors contributions
Shabana Naz: conceptualization, validation
Rida Nadeem: sampling and data analysis, investigation
Gulnaz Bibi: Methodology and conceptualization, data curation
Ibrahim A. Alhidary: drafting manuscript, review, visualization
Rifat Ullah Khan: Drafting and Editing manuscript and submission
Dai Sifa: data curation, Final proofs reading and editing
Ethical approval
The Committee on Animal Rights and Welfare, GC University Faisalabad, Pakistan approved this study (DZ/34/2023)
Consent to participate
All authors are agreed to submit the article to this journal
Consent to publish
All authors are agreed to publish in this journal
Acknowledgement
We extend our appreciation to the Researchers Supporting Project (No. RSPD2024R833), King Saud University, Riyadh, Saudi Arabia
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
On request.
Additional information
Funding
References
- Ahmadi M, Alves BXR, Baker CJ, Bertsche W, Capra A, Carruth C, Cesar CL, Charlton M, Cohen S, Collister R, et al. 2018. Characterization of the 1S–2S transition in antihydrogen. Nature. 557(7703):71–75. doi: 10.1038/s41586-018-0017-2.
- Akhtar MJ, Kumar S, Alhadlaq HA, Alrokayan SA, Abu-Salah KM, Ahamed M. 2016. Dose-dependent genotoxicity of copper oxide nanoparticles stimulated by reactive oxygen species in human lung epithelial cells. Toxicol Ind Health. 32(5):809–821. doi: 10.1177/0748233713511512.
- Alagawany M, Qattan SYA, Attia YA, El-Saadony MT, Elnesr SS, Mahmoud MA, Madkour M, Abd El-Hack ME, Reda FM. 2021. Use of chemical nano-selenium as an antibacterial and antifungal agent in quail diets and its effect on growth, carcasses, antioxidant, immunity and caecal microbes. Animals. 11(11):3027. doi: 10.3390/ani11113027.
- Aslam B, Hussain A, Faisal MN, Sindhu ZD, RU Khan, Alhidary IA, Naz S, Tufarelli V. 2023. Curcumin coencapsulation potentiates anti-arthritic efficacy of meloxicam biodegradable nanoparticles in adjuvant-induced arthritis animal model. Biomedicines. 11:2662. https://doi.org/10.3390/biomedicines11102662
- Auon M, Mahmood F, Khan A, Hussain R. 2014. Testicular and genotoxic effects induced by subchronic oral administration of chlorpyrifos in Japanese quail (Coturnix japonica). Pak J Agric Sci. 51(4):158–167.
- Bami MK, Afsharmanesh M, Espahbodi M, Esmaeilzadeh E. 2022. Effects of dietary nano-selenium supplementation on broiler chicken performance, meat selenium content, intestinal microflora, intestinal morphology, and immune response. J Trace Elem Med Biol. 69(5):126897–126899. doi: 10.1016/j.jtemb.2021.126897.
- Aziz S, Abdullah S, Anwar H, Latif F. 2022. DNA damage and oxidative stress in economically important fish, bighead carp (Hypophthalmichthys nobilis) exposed to engineered copper oxide nanoparticles. Pak Vet J. 42(1):1–8.
- Batra G, Gortzi O, Lalas SI, Galidi A, Alibade A, Nanos GD. 2017. Enhanced antioxidant activity of Capsicum annuum L. and Moringa oleifera L. extracts after encapsulation in microemulsions. Chem Eng. 1(2):15–18. doi: 10.3390/chemengineering1020015.
- Boostani A, Sadeghi AA, Mousavi SN, Chamani M, Kashan N. 2015. Effects of organic, inorganic, and nano-Se on growth performance, antioxidant capacity, cellular and humoral immune responses in broiler chickens exposed to oxidative stress. Livest Sci. 178:330–336. doi: 10.1016/j.livsci.2015.05.004.
- Chen N, Yao P, Zhang W, Zhang Y, Xin N, Wei H, Zhao C. 2022. Selenium nanoparticles: enhanced nutrition and beyond. Crit Rev Food Sci Nutr. 63(4):1–12.
- Dang F, Huang Y, Wang Y, Zhou D, Xing B. 2021. Transfer and toxicity of silver nanoparticles in the food chain. Environ Sci Nano. 8(6):1519–1535. doi: 10.1039/D0EN01190H.
- Dawood MA, Zommara M, Eweedah NM, Helal AI, Aboel-Darag MA. 2020. The potential role of nano-selenium and vitamin C on the performances of Nile tilapia (Oreochromis niloticus). Environ Sci Pollut Res Int. 27(9):9843–9852. doi: 10.1007/s11356-020-07651-5.
- De Jong WH, De Rijk E, Bonetto A, Wohlleben W, Stone V, Brunelli A, Badetti E, Marcomini A, Gosens I, Cassee FR. 2019. Toxicity of copper oxide and basic copper carbonate nanoparticles after short-term oral exposure in rats. Nanotoxicology. 13(1):50–72. doi: 10.1080/17435390.2018.1530390.
- Eid YZ, Omara Y, Ragab A, Ismail A, Zommara M, Dawood MA. 2023. Mitigation of imidacloprid toxicity in poultry chicken by selenium nanoparticles: growth performance, lipid peroxidation, and blood traits. Biol Trace Elem Res. 201(11):5379–5388. doi: 10.1007/s12011-023-03592-5.
- Eid YZ, Zomara M, Tawfeek FA. 2022. Effect of the biologically produced nanoselenium dietary supplementation on growth performance, carcass characteristics, blood parameters, and economic efficiency in broiler chickens. AJVS. 73(2):47–47. doi: 10.5455/ajvs.44970.
- Eldeeb F, Ibrahim HH. 2023. Impact of adding different levels of nanoselenium to duck diets on growth performance, immunity status, antioxidant activity and some blood parameters. J Adv Vet Res. 13(8):1642–1649.
- El-Kazaz SE, Abo-Samaha MI, Hafez MH, El-Shobokshy SA, Wirtu G. 2020. Dietary supplementation of nano-selenium improves reproductive performance, sexual behavior and deposition of selenium in the testis and ovary of Japanese quail. J Adv Vet Anim Res. 7(4):597–607. doi: 10.5455/javar.2020.g457.
- Fotouh A, Gab-Allah MS, Tantawy AA, Soufy H, Nasr SM. 2014. Alterations of blood components in broiler chicks experimentally infected with Salmonella Gallinarum. Global Veterinaria. 13:787–793.
- Foroutankhah M, Toghyani M, Landy N. 2019. Evaluation of Calendula officinalis L.(marigold) flower as a natural growth promoter in comparison with an antibiotic growth promoter on growth performance, carcass traits and humoral immune responses of broilers. Anim Nutr. 5(3):314–318. doi: 10.1016/j.aninu.2019.04.002.
- Hassan MA, Shehabeldin A, Omar M, Khalil WA, Swelum AA, Lu Y, Abdelnour SA. 2023. Effect of spirulina nanoparticles or selenium-coated spirulina nanoparticles supplemented to freezing extender on bull sperm freezability. Pak Vet J. 43(4):739–747.
- Hussain R, Mahmood F, Khan MZ, Khan A, Muhammad F. 2011. Pathological and genotoxic effects of atrazine in male Japanese quail (Coturnix japonica). Ecotoxicology. 20(1):1–8. doi: 10.1007/s10646-010-0515-y.
- Ifijen IH, Atoe B, Ekun RO, Ighodaro A, Odiachi IJ. 2023. Treatments of Mycobacterium tuberculosis and Toxoplasma gondii with selenium nanoparticles. Bionanoscience. 13(1):249–277. doi: 10.1007/s12668-023-01059-4.
- Jamima J, Veeramani P, Kumanan K, Kanagaraju P. 2020. Production performance, hematology and serum biochemistry of commercial broilers supplemented with nano selenium and other anti-stressors during summer. IJAR. 54(of):1385–1390. doi: 10.18805/ijar.B-3902.
- Jiang Z, Lin Y, Zhou G, Luo L, Jiang S, Chen F. 2009. Effects of dietary selenomethionine supplementation on growth performance, meat quality and antioxidant property in yellow broilers. J Agric Food Chem. 57(20):9769–9772. doi: 10.1021/jf902411c.
- Karlsson HL. 2010. The comet assay in nanotoxicology research. Anal Bioanal Chem. 398(2):651–666. doi: 10.1007/s00216-010-3977-0.
- Kassim AS, Ali AHH, Marwan TA, Abdel-Wareth AAA. 2022. Selenium nanoparticles in rabbit nutrition. A review. SVU-IJAS. 4(1):90–98. doi: 10.21608/svuijas.2022.117298.1171.
- Khalaf AA, Zaki AR, Galal MK, Ogaly HA, Ibrahim MA, Hassan A. 2017. The potential effect of alpha-liponic acid against nanocopper particle-induced hepatotoxicity in male rates. Hum Exp Toxicol. 36(9):881–891. doi: 10.1177/0960327116674526.
- Khan I, Zaneb H, Masood S, Ashraf S, Rehman HF, Rehman HU, Ahmad S, Taj R, Rahman SU. 2022. Supplemental selenium nanoparticles-loaded to chitosan improves meat quality, pectoral muscle histology, tibia bone morphometry and tissue mineral retention in broilers. Pak Vet J. 42(2):236–240.
- Kieliszek M, Błażejak S. 2013. Selenium: significance, and outlook for supplementation. Nutrition. 29(5):713–718. doi: 10.1016/j.nut.2012.11.012.
- Kousar S, Javed M. 2015. Diagnosis of metals induced DNA damage in fish using comet assay. Pak Vet J. 35(2):168–172.
- Kumar A, Prasad KS. 2021. Role of nano-selenium in health and environment. J Biotechnol. 325(3):152–163. doi: 10.1016/j.jbiotec.2020.11.004.
- Landy N, Kheiri F, Faghani M. 2020. Evaluation of cottonseed bioactive peptides on growth performance, carcase traits, immunity, total antioxidant activity of serum and intestinal morphology in broiler chickens. Ital J Anim Sci. 19(1):1375–1386. doi: 10.1080/1828051X.2020.1844085.
- Lin W, Xu Y, Huang CC, Ma Y, Shannon KB, Chen DR, Huang YW. 2009. Toxicity of nano-and micro-sized ZnO particles in human lungs epithelial cells. J Nanopart Res. 11(1):25–39. doi: 10.1007/s11051-008-9419-7.
- Malyugina S, Skalickova S, Skladanka J, Slama P, Horky P. 2021. Biogenic selenium nanoparticles in animal nutrition: a review. Agriculture. 11(12):1244. doi: 10.3390/agriculture11121244.
- Masour ES, Hh A, A, Shawky N, El RasHidy R, S H A. 2014. Studies on effect of salmonella pullorum in balady chicks and its treatment with doxycycline. Zagazig Vet J. 42(3):145–156. doi: 10.21608/zvjz.2014.60061.
- Medina Cruz D, Mi G, Webster TJ. 2018. Synthesis and characterization of biogenic selenium nanoparticles with antimicrobial properties made by Staphylococcus aureus, methicillin-resistant Staphylococcus aureus (MRSA), Escherichia coli, and Pseudomonas aeruginosa. J Biomed Mater Res A. 106(5):1400–1412. doi: 10.1002/jbm.a.36347.
- Mohamed HS, Rizk YS, Elslamony AE, Soliman AA, Ebrahim AF. 2016. Study the relationship between selenium and heat shock proteins under heat stress for local sinai chickens strain. EPSJ. 36(1):337–354. doi: 10.21608/epsj.2016.33379.
- Monteith AJ, Skaar EP. 2021. The impact of metal availability on immune function during infection. Trends Endocrinol Metab. 32(11):916–928. doi: 10.1016/j.tem.2021.08.004.
- Morsy MK, Khalaf HH, Sharoba AM, El-Tanahi HH, Cutter CN. 2014. Incorporation of essential oils and nanoparticles in pullulan films to control foodborne pathogens on meat and poultry products. J Food Sci. 79(4):M675–M684. doi: 10.1111/1750-3841.12400.
- Naz S, Raza N, Alhidary I, Satti S, Rafique A, Batool S, Shamsi S, Dai S, RU Khan. 2024. Evaluation of copper nanoparticles on growth, organs histology and DNA damage in Japanese quails (Coturnix coturnix japonica). Toxin Reviews. doi: 10.1080/15569543.2023.2298909.
- Nikdehghan N, Kashiri H, Hedayati AA. 2018. CuO nanoparticles-induced micronuclei and DNA damage in Cyprinus carpio. AACL Bioflux. 11(3):112–116.
- Ostovar M, Saberi N, Ghiassi R. 2022. Selenium contamination in water; analytical and removal methods: a comprehensive review. Sep Sci Technol. 57(15):2500–2520. doi: 10.1080/01496395.2022.2074861.
- Reda FM, El-Saadony MT, Elnesr SS, Alagawany M, Tufarelli V. 2020. Effect of dietary supplementation of biological curcumin nanoparticles on growth and carcass traits, antioxidant status, immunity and caecal microbiota of Japanese quails. Animals. 10(5):754. doi: 10.3390/ani10050754.
- Reda FM, El-Saadony MT, El-Rayes TK, Attia AI, El-Sayed SAA, Ahmed SYA, Madkour M, Alagawany M. 2021. Use of biological nano zinc as a feed additive in quail nutrition: biosynthesis, antimicrobial activity and its effect on growth, feed utilisation, blood metabolites and intestinal microbiota. Ital J Anim Sci. 20(1):324–335. doi: 10.1080/1828051X.2021.1886001.
- Sa’aci ZA, Jiya EZ, Ijaiya AT. 2021. Influence of dietary nano zinc and selenium supplementation on growth performance, nutrients digestibility and carcass characteristics of broiler chickens. Niger J Anim Sci. 23(3):185–198.
- Saleh AA, Ebeid TA. 2019. Feeding sodium selenite and nano-selenium stimulates growth and oxidation resistance in broilers. SA J Anim Sci. 49(1):176–183. doi: 10.4314/sajas.v49i1.20.
- Singh NP, McCoy MT, Tice RR, Schneider EL. 1988. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 175(1):184–191. doi: 10.1016/0014-4827(88)90265-0.
- Wang Y, Aker WG, Hwang HM, Yedjou CG, Yu H, Tchounwou PB. 2011. A study of the mechanism of in vitro cytotoxicity of metal oxide nanoparticles using catfish primary hepatocytes and human HepG2 cells. Sci Total Environ. 409(22):4753–4762. doi: 10.1016/j.scitotenv.2011.07.039.
- Yang H, Liu C, Yang D, Zhang H, Xi Z. 2009. Comparative study of cytotoxicity, oxidative stress and genotoxicity induced by four typical nanomaterial: the role of particle size, shape and composition. J Appl Toxicol. 29(1):69–78. doi: 10.1002/jat.1385.
- Zambonino MC, Quizhpe EM, Jaramillo FE, Rahman A, Santiago Vispo N, Jeffryes C, Dahoumane SA. 2021. Green synthesis of selenium and tellurium nanoparticles: current trends, biological properties and biomedical applications. Int J Mol Sci. 22(3):989. doi: 10.3390/ijms22030989.