ABSTRACT
Nitrous oxide is used as an anaesthetic and analgesic agent in the medical setting and is known to cause raised intracranial pressure. The use of nitrous oxide recreationally for the drug’s euphoric and relaxant properties has been linked to multiple neurological and psychiatric sequelae including neuropathy, myelopathy, and psychosis. We describe a case of a young person who declared heavy nitrous oxide use resulting in vision-threatening papilloedema secondary to raised intracranial pressure. He underwent emergency lumbar drainage alongside high-dose acetazolamide and parenteral vitamin B12 injections. To our knowledge, there have yet to be other reports of cases where heavy nitrous oxide use has caused secondary pseudotumor cerebri syndrome.
Introduction
Nitrous oxide is commonly used in the medical and dental setting as an inhalational anaesthetic agent. However, it was used recreationally for public entertainment and in British high society parties as early as the 18th century, long before its medical potential was recognised.Citation1 There has been a recent resurgence of recreational use of the gas. In the United Kingdom, it is the second most prevalent drug of abuse after cannabis.Citation2 Apart from its haematological and cardiorespiratory risks, its neurological manifestations in the form of subacute combined degeneration of the cord and motor and sensory neuropathies are most well known. The commonest presenting neurological issues in patients with chronic nitrous oxide use are sensory ataxia and abnormal vibration and proprioception, with posterior and posterolateral column dysfunction.Citation3 There have been no reports of visual loss in patients with recreational nitrous oxide use in the current English literature. We describe a case of vision-threatening papilloedema in a young person with a background of chronic, high dose recreational nitrous oxide use.
Case report
A right-handed male student living with obesity who was in his early twenties presented 1-week following a thunderclap headache. He reported to be halfway through a nitrous oxide canister when he was suddenly affected by an intense severe headache, coming on instantly from zero to maximal pain. At the time, he was not exerting himself other than inhaling a bag of nitrous oxide. He reported that his vision became blurred and he saw non-coloured flashing lights in both eyes. He was a heavy recreational user of nitrous oxide, using balloon delivery with an estimated 0.5 litre canister daily in the preceding 4 months. He was otherwise well with no prior personal or family history of migraine and no significant past medical or family history.
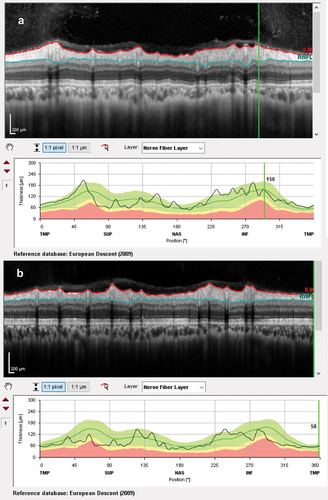
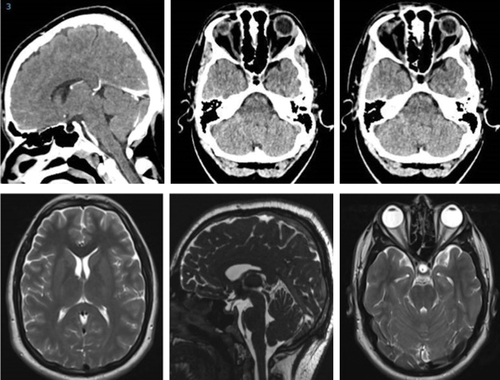
At presentation, he had a moderately severe, constant, diffuse headache with photophobia and phonophobia. On examination, there were no focal neurological deficits. He had a body mass index of 32 kg/m2. He had reduced visual acuity of 6/12 in his right eye and 6/18 in his left eye with a left relative afferent pupillary defect. Colour vision using Ishihara plates was normal in each eye. The visual fields to confrontation showed bilateral enlarged blind spots and a left inferior nasal step. His intraocular pressures were: right eye 13 mmHg and left eye 15 mmHg. Dilated slit-lamp examination revealed symmetrical Frisén grade 5 papilloedema with haemorrhages and cotton wool spots, suggestive of mixed compressive and ischaemic optic neuropathy. His optical coherence tomography (OCT) images quantitatively revealed the extent of the papilloedema, with global peripapillary retinal nerve fibre layer (RNFL) thicknesses of 466 µm in the right eye and 437 µm in the left (). Computed tomography (CT) scanning and magnetic resonance imaging (MRI) of the head showed a partially empty sella turcica, distortion of the optic nerves and narrowing of the transverse sinuses; features suggestive of intracranial hypertension (). CT venography did not show a venous sinus thrombosis. MRI of the whole spine (which included the cervical, thoracic, and lumbar regions) was unremarkable. Lumbar puncture opening pressure was 51 cm of cerebrospinal fluid (CSF). CSF analysis was unremarkable. Serum inflammatory markers, haemoglobin, coagulation profile, and vitamin B12 levels were normal.
Figure 1. Optical coherence tomography (OCT) infrared images of the optic nerves at presentation: (a) right eye; (b) left eye, both showing papilloedema. OCT infra-red images at 2 months, following treatment: (c) right eye; (d) left eye, both without papilloedema.
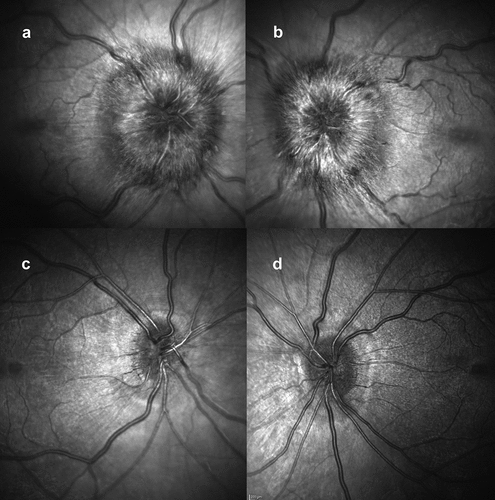
Figure 2. Optical coherence tomography retinal nerve fibre layer thickness (RNFL) at presentation showing increased thicknesses of the RNFL: (a) right eye and (b) left eye.
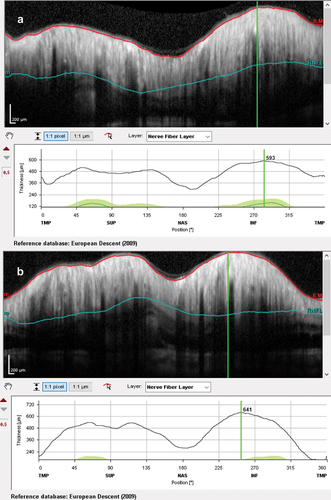
Figure 3. Computed tomography scans (upper row) and magnetic resonance imaging (lower row) showing a partially empty sella turcica and small ventricles, suggestive of intracranial hypertension.
He underwent emergency lumbar drainage and was started on an escalating dose of acetazolamide.Citation4 He was also prescribed parenteral vitamin B12 injections for suspected nitrous oxide-induced functional vitamin B12 deficiency (the initial blood sample had been insufficient to confirm vitamin B12 deficiency). Subsequent biochemistry tests for this were normal: the serum homocysteine level was 13 µmol/L (laboratory reference <18 µmol/L); methylmalonic acid level was requested, however the laboratory reported their test was not sufficiently sensitive for the investigation of vitamin B12 deficiency. After 2 days, the drain was removed against medical advice. Following discussions regarding treatment options for continued sight-threatening disease, such as a permanent CSF shunt or optic nerve sheath fenestration, the drain was re-sited for a further 5 days.
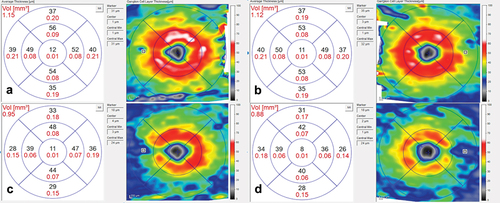
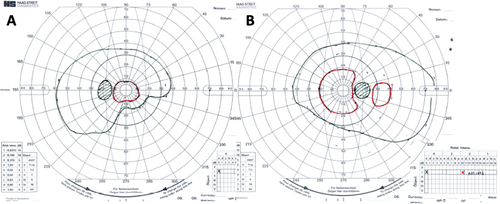
Over the course of his 2-week admission, the papilloedema settled and his vision and headaches improved. At discharge, his visual acuity was 6/5 in each eye. His relative afferent pupillary defect had resolved, and his visual field had subjectively improved (). He was advised to abstain from nitrous oxide use and reduce the acetazolamide from 2 g in a daily divided dose to stopping over the next 2 months. At 2 months, the papilloedema had resolved with global peripapillary RNFL thicknesses of 102 µm in the right eye and 81 µm in the left eye (). His macular ganglion cell layer at 2 months was abnormal with specific loss in the temporal macular regions, worse in the left eye ().
Figure 4. Goldmann visual fields in the (a) Left eye and (b) Right eye at 2 months, following treatment.
Discussion
The recreational use of nitrous oxide, especially in young adults, has been rising in recent years. In the short term, it results in brief dissociative, euphoric and hallucinogenic effects and sedation. These effects are thought to be mediated through N-methyl-D-aspartate (NMDA) antagonism, decreasing excitatory neurotransmission by non-competitive glutamate inhibition.Citation5 Nitrous oxide is also a partial mu, kappa, and delta opioid receptor agonist, which may be responsible for its analgesic effect.Citation6 Chronic nitrous oxide exposure results in dose-dependent inactivation of vitamin B12, a co-factor required for methionine synthesis as well as direct inhibition of the enzyme methionine synthase.Citation7 Methionine is required for deoxyribonucleic acid synthesis and maintenance of myelin sheaths. ‘Functional’ vitamin B12 deficiency secondary to nitrous oxide abuse has been associated with demyelination within the central and peripheral nervous systems.Citation8 Other effects of nitrous oxide-induced dysfunctional B12 metabolism include agranulocytosis, megaloblastic anaemia with bone marrow suppression, subacute combined degeneration of the spinal cord and reversible psychosis.Citation7 In our case, homocysteine levels were normal, suggesting no functional vitamin B12 deficiency, which implied that the visual loss was not secondary to chronic nutritional optic neuropathy.
While neuropathy secondary to nitrous oxide use has been a recognised phenomenon since the 1970s,Citation9 there have been no reports of recreational nitrous oxide use-associated raised intracranial pressure and associated visual loss. This is the first case we could identify linking recreational nitrous oxide use to fulminant papilloedema. High dose nitrous oxide induced intracranial pressure (ICP) elevation is a well-known phenomenon in the context of induction of anaesthesia with studies showing a mean increase of up to 27 mmHg. The most likely mechanism for this is thought to be cerebral vasodilation leading to increased intracranial blood volume, with quick reversibility in ICP once nitrous oxide is withdrawn.Citation10,Citation11 Inhalation of 50% nitrous oxide has also been shown to increase middle cerebral artery blood flow velocity and to impair cerebral autoregulation in healthy people. It is possible that such blood flow variations and loss of autoregulation in the context of chronic high dose nitrous oxide abuse could have triggered a prolonged episode, as demonstrated here. As nitrous oxide was the likely known initiator of this event, it was deemed reasonable to offer a temporary lumbar drain rather than a longer-term CSF shunt.
The thunderclap headache presentation was unusual for raised ICP.Citation12 While some could consider this presentation secondary to the drug effect, nitrous oxide is a vasodilator rather than a vasoconstrictor; therefore, this event was likely not attributed to the reversible cerebral vasoconstriction syndrome. The pseudotumor cerebri syndrome can be primary or secondary, both of which have different risk factors. For example, the main risk factor for primary pseudotumor cerebri syndrome, or idiopathic intracranial hypertension (IIH), is weight gainCitation13 and while our case was in the obesity category, we cannot be certain that this did not in some way contribute to the mechanism of the raised ICP. Anaemia is a recognised risk factor for secondary pseudotumor cerebri, with up to 10% of cases with ICP having iron deficiency anaemia.Citation14 Reynolds et al.Citation15 carried out a systematic review to identify other nutrient deficiencies which may be linked to optic disc swelling – vitamin B12 deficiency was found to be linked to optic disc swelling in 11 cases, with two of the 11 cases also meeting the diagnostic criteria for IIH. In all cases, vitamin B12 deficiency was a result of poor intake, rather than functional deficiency from a toxic cause. In all cases, anaemia, if present, was not severe, suggesting that vitamin B12 deficiency may cause neurological dysfunction independent of haemoglobin levels. Vitamin B12 deficiency without anaemia resulting in papilloedema has been demonstrated in other case series as well.Citation16–18 The mechanism for this is unclear. Perhaps, methionine deficiency directly results in toxic ischaemic optic neuropathy triggering optic disc swelling as an early feature. Additionally, in the context of pseudotumor cerebri, it is yet to be explored what role nutrition and vitamin B12 deficiency may have in causing metabolic derangement contributing to increased CSF secretion. Lastly, homocysteinaemia, which results from accumulation of homocysteine in functional vitamin B12 deficiency, and lipoprotein a in conjunction with this, have been associated with venous sinus thrombosis, a known cause of intracranial hypertension. In our case, there was no radiological evidence of venous sinus thrombosis, nor evidence of raised homocysteine levels.
Physicians should also be vigilant to nitrous oxide use as a potential triggering factor for raised ICP and visual loss, additionally querying whether nutritional deficiency or malabsorption of vitamin B12 may have a role to play in the clinical presentation. A challenge in this case was that insufficient blood samples were received on admission that would have permitted a diagnosis of vitamin B12 deficiency prior to starting replacement therapy. In our case, a combination of lumbar drainage and acetazolamide was adopted as successful mechanisms to manage vision-threatening pseudotumour cerebri syndrome.
Conclusion
The neurological sequelae of chronic nitrous oxide use are well known, however it is only in recent years that the health implications have gained recognition among public health authorities, healthcare providers, and consumers. It is biologically plausible in the context of known neurotoxic effects of nitrous oxide that there exists a link between chronic nitrous oxide use and the development of severe papilloedema and vision-threatening pseudotumour cerebri as seen in this case. Clinicians should consider whether nitrous oxide abuse may be relevant risk factor in the context of a person who presents with papilloedema.
Ethical approval
We have obtained written consent to publish this case.
Disclosure statement
Professor Susan Mollan has received honoria for teaching from Novartis (2019); Chiesi (2020,2021), Heidelberg Engineering (2019, 2020,2021), and Teva (2019, 2021). Professor Mollan has served on advisory boards for Invex Therapeutics Janssen, and Gensight in the last 3 years. Professor Mollan has received payment for consultancy work for Invex therapeutics (2020, 2021, 2023).
All other authors have no disclosures to declare.
The authors alone are responsible for the content and writing of the paper.
Additional information
Funding
References
- Randhawa G, Bodenham A. The increasing recreational use of nitrous oxide: history revisited. Br J Anaesth. 2016;116(3):321–324. doi:10.1093/bja/aev297.
- van Amsterdam J, Nabben T, van den Brink W. Recreational nitrous oxide use: prevalence and risks. Regul Toxicol Pharmacol. 2015;73(3):790–796. doi:10.1016/j.yrtph.2015.10.017.
- Lan SY, Kuo CY, Chou CC, et al. Recreational nitrous oxide abuse related subacute combined degeneration of the spinal cord in adolescents – a case series and literature review. Brain Dev. 2019;41(5):428–435. doi:10.1016/j.braindev.2018.12.003.
- Mollan SP, Davies B, Silver NC, et al. Idiopathic intracranial hypertension: consensus guidelines on management. J Neurol Neurosurg Psychiatry. 2018;89(10):1088–1100. doi:10.1136/jnnp-2017-317440.
- Jevtović-Todorović V, Todorović SM, Mennerick S, et al. Nitrous oxide (laughing gas) is an NMDA antagonist, neuroprotectant and neurotoxin. Nat Med. 1998;4(4):460–463. doi:10.1038/nm0498-460.
- Gillman MA, Lichtigfeld FJ. Clinical role and mechanisms of action of analgesic nitrous oxide. Int J Neurosci. 1998;93(1–2):55–62. doi:10.3109/00207459808986412.
- Winstock AR, Ferris JA. Nitrous oxide causes peripheral neuropathy in a dose dependent manner among recreational users. J Psychopharmacol. 2020;34(2):229–236. doi:10.1177/0269881119882532.
- Mair D, Paris A, Zaloum SA, et al. Nitrous oxide-induced myeloneuropathy: a case series. J Neurol Neurosurg Psychiatry. 2023;94(9):681–688. doi:10.1136/jnnp-2023-331131.
- Layzer RB, Fishman RA, Schafer JA. Neuropathy following abuse of nitrous oxide. Neurology. 1978;28(5):504–506. doi:10.1212/wnl.28.5.504.
- Phirman JR, Shapiro HM. Modification of nitrous oxide-induced intracranial hypertension by prior induction of anesthesia. Anesthesiology. 1977;46(2):150–151. doi:10.1097/00000542-197702000-00017.
- Henriksen HT, Jörgensen PB. The effect of nitrous oxide on intracranial pressure in patients with intracranial disorders. Br J Anaesth. 1973;45(5):486–492. doi:10.1093/bja/45.5.486.
- Mollan SP, Spitzer D, Nicholl DJ. Raised intracranial pressure in those presenting with headache. BMJ. 2018;363:k3252. doi:10.1136/bmj.k3252.
- Mollan SP, Tahrani AA, Sinclair AJ. The potentially modifiable risk factor in idiopathic intracranial hypertension: body weight. Neur Clin Pract. 2021;11(4):e504–e507. doi:10.1212/CPJ.0000000000001063.
- Mollan SP, Ball AK, Sinclair AJ, et al. Idiopathic intracranial hypertension associated with iron deficiency anaemia: a lesson for management. Eur Neurol. 2009;62(2):105–108. doi:10.1159/000222781.
- Reynolds G, Epps S, Huntley A, Atan D. Micronutrient deficiencies presenting with optic disc swelling associated with or without intracranial hypertension: a systematic review. Nutrients. 2022;14(15):3068. doi:10.3390/nu14153068.
- Lindenbaum J, Healton EB, Savage DG, et al. Neuropsychiatric disorders caused by cobalamin deficiency in the absence of anemia or macrocytosis. N Engl J Med. 1988;318(26):1720–1728. doi:10.1056/NEJM198806303182604.
- Biousse V, Rucker JC, Vignal C, Crassard I, Katz BJ, Newman NJ. Anemia and papilledema. Am J Ophthalmol. 2003;135(4):437–446. doi:10.1016/s0002-9394(02)02062-7.
- Yetgin S, Derman O, Dogan M. A pediatric patient with recurrent pseudotumor cerebri and vitamin B12 deficiency. Pediatr Hematol Oncol. 2006;23(1):39–43. doi:10.1080/08880010500313322.