ABSTRACT
Background
Traditionally, surgical removal of glioblastoma is performed with general anaesthesia but a recent meta-analysis revealed that awake surgery in glioblastoma resulted in better surgical outcomes than non-awake surgery. Preoperative severe aphasia is one of the exclusion criteria for awake surgery because of difficulties in intraoperative interpretation of language deterioration and the distinction between preoperative vs. intraoperative induced paraphasias. As severe aphasia is common in glioblastoma patients, many potential patients who may benefit from awake surgery are excluded.
Aims
We aim to investigate the feasibility of awake surgery in glioblastoma patients with severe aphasia using a patient-tailored approach with adapted intraoperative language tasks. We also examined the effect of awake surgery on language outcomes.
Methods & Procedures
We discuss five case studies of patients elected for awake surgery with presumed glioblastoma in eloquent language areas and severe aphasia. Pre- and postoperatively, an extensive test-protocol was administered at different linguistic levels and modalities. A patient-tailored intraoperative language test-protocol was applied.
Outcomes & Results
Preoperatively, all patients had severe impairments on all language and cognitive tests. Intraoperative language tasks for direct electrical stimulation and resection were selected and adapted to patients’ preoperative level. Despite preoperative severe aphasia, functional boundaries for critical language areas could be identified in each patient. Postoperatively, all patients had stable or improved language outcome. One of the patients recovered to maximum scores on nearly all language tests.
Conclusions
Our cases demonstrate that awake surgery in severely aphasic glioblastoma patients is feasible and did not cause further deterioration of aphasia. An extensive preoperative neurolinguistic examination is necessary for adequate patient-tailored intraoperative monitoring with maximal tumour resection and to consequently increase the chance of language preservation and quality of life.
Introduction
Glioblastomas are malignant primary brain tumours with a limited median survival of 12-15 months (Stupp et al., Citation2005). Traditionally, surgical removal of glioblastoma is performed with general anaesthesia with the goal of achieving a maximal safe resection. Postoperatively, some studies report no significant deterioration of cognitive and language functions (Dallabona et al., Citation2017; Habets et al., Citation2014), although others reported both improvement and deterioration (McGirt et al., Citation2009; Rahman et al., Citation2017; Sinha et al., Citation2020; Whittle et al., Citation1998).
In contrast, in low-grade glioma in eloquent areas, maximal extent of resection using direct electrical stimulation (DES) during awake surgery is considered the gold standard treatment to avoid irreversible neurological and cognitive damage (De Witt Hamer et al., Citation2012). The usefulness of awake surgery and its impact on neurological outcome has been evaluated sparsely in glioblastoma but so far, there seems to be a trend towards a higher percentage of total resection, a lower complication rate and a longer median survival than after surgery with general anaesthesia (Gerritsen et al., Citation2019; Gerritsen et al., Citation2022; Li et al., Citation2021). Also language and cognition can be better preserved during awake surgery than with general anaesthesia (Bonifazi et al., Citation2020; van Kessel et al., Citation2020; Zigiotto et al., Citation2020).
Cognitive deficits including language impairments are preoperatively more prevalent and significantly worse in glioblastoma patients than in low-grade glioma patients (IJzerman-Korevaar et al., Citation2018; Noll et al., Citation2015; van Kessel et al., Citation2017; van Kessel et al., Citation2019). Preoperative severe aphasia is one of the exclusion criteria for awake surgery because of difficulties in interpretation of intraoperative language deterioration and the distinction between preoperative vs. intraoperative induced paraphasias (Bonifazi et al., Citation2020; Dziedzic & Bernstein, Citation2014; Zigiotto et al., Citation2020). Since the incidence of aphasia in glioblastoma patients in the diagnostic phase is 16-58% (Dallabona et al., Citation2017; IJzerman-Korevaar et al., Citation2018; McGirt et al., Citation2009; Tandon et al., Citation1993), many potential patients who may benefit from awake surgery are excluded.
Over the last decade, language testing in the pre-, intra- and postoperative phase of glioma surgery became part of standard care (Rofes et al., Citation2017; Sierpowska et al., Citation2022). In addition, apart from object naming, a larger variety of language tests for intraoperative monitoring is nowadays available which may facilitate intraoperative language monitoring in more severe aphasic population (De Witte & Marien, Citation2013; De Witte et al., Citation2015; Rofes & Miceli, Citation2014).
In this study, we aimed to investigate whether awake surgery in patients with glioblastoma in eloquent language areas suffering from severe preoperative aphasia is feasible. The following research questions were investigated:
Could in awake surgery of glioblastoma patients with severe aphasia intraoperative language deterioration be distinguished from preoperative aphasia when a neurolinguistic patient-tailored approach is used with language tasks adapted to the preoperative language level?
What is the language outcome after awake surgery of severely aphasic glioblastoma patients?
We hypothesise that awake surgery in glioblastoma patients with severe aphasia is possible when using adapted intraoperative language tests taking into account linguistic variables (e.g., word frequency, word length, age of acquisition). By doing so, a distinction can be made between pre-existent paraphasias and DES induced paraphasias. We expect that with this technique a stable postoperative language outcome (or even improvement) can be obtained.
Materials and methods
Participants
We present five case studies of patients who were retrospectively selected for this study and underwent awake surgery based on tumour location in eloquent language areas. The patients (A1, B2, C3, D4 and E5) were diagnosed with a presumed glioblastoma in the language dominant left hemisphere as identified on MRI (see ) and with preoperative severe aphasia. The tumours were localized in frontal areas in A1 and B2, temporal in C3 and temporo-parietal in D4 and E5. D4 and E5 suffered from a recurrent glioblastoma that was treated earlier with surgery with general anaesthesia twice (D4 36 and 45 months, E5 26 and 82 months before current surgery) and chemo-radiation. Patients’ demographic and clinical characteristics are presented in . The Ethical Committee of Erasmus MC Rotterdam approved the study which waived the need for written consent from the patients because of the retrospective nature of the study and the emotional burden that would result from contacting the patients or their relatives to obtain consent.
Figure 1. Pre- and postoperative post-contrast T1-weighted MRI scans in the axial and sagittal plane in patients A1, B2, C3, D4 and E5. Pre = preoperative. Post = postoperative
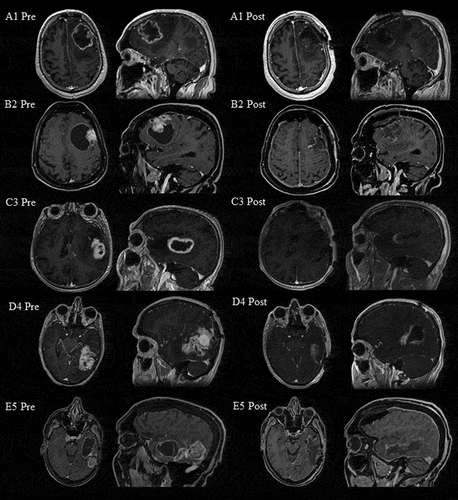
Table 1. Demographic and clinical characteristics.
Procedure: operation, neuroimaging and pathological findings
Patients were treated in Erasmus MC and HMC between November 2017 and September 2019 in an awake setting after administration of local anaesthesia. DES with a bipolar electrode was carried out at cortical and subcortical level to identify individual functional boundaries while word repetition or object naming were administered. Several language tasks at the linguistic levels of phonology, semantics and syntax from the Dutch Linguistic Intraoperative Protocol (DuLIP) (De Witte et al., Citation2015) relevant in the operated cortico-subcortical areas in combination with spontaneous speech monitoring were conducted (see Supplementary material Figure 1-6). Due to preoperative severe aphasia in all patients, the selected DuLIP-tasks needed to be patient-tailored and simplified, so that new intraoperative occurrences of neologisms, perseverations and/or paraphasias could be interpreted as further language deterioration. Due to a lack of semantic tasks for severe aphasia in DuLIP, two new semantic judgment tasks were developed (see Supplementary material Figure 5 and 6). Resection was ended when the tumour was macroscopically completely removed or when language deteriorated significantly compared to preoperative level.
Localization and delineation of the tumour was determined by a neuroradiologist using contrast-enhanced 3D T1-weighted MRI-images. The pre- and postoperative tumour volumes were calculated by semi-automated delineation of the contrast-enhanced area, with inclusion of any enclosed areas of necrosis, using ITK-SNAP version 3.6.0 (www.itksnap.org, Yushkevich et al. (Citation2006)). Postoperative MRI-scans were acquired within 48 hours after surgery. The extent of resection was calculated as the fraction of the difference between the pre- and postoperative volume divided by the preoperative volume. The histological and molecular type of the tumour were determined by a neuropathologist from tissue obtained during the resection.
Neurolinguistic assessment
Pre- and postoperatively, an extensive neurolinguistic test-protocol and a cognitive screening () was administered by the authors DS, CDK or MDK. They are the speech and language pathologists (SLP) who also conducted the intraoperative monitoring. Tests were administered a week before surgery. When extra information is needed, additional tests were administered a day before surgery. At the start of the intraoperative awake phase (i.e., before DES), the baseline level was screened again. The neurolinguistic protocol was patient-tailored and consisted of tasks at different linguistic levels (phonology, semantics and syntax) and modalities (production, comprehension and reading). The core of the neurolinguistic protocol were the shortened Token Test (TT, De Renzi and Faglioni (Citation1978)), Boston Naming Test (BNT, Kaplan et al. (Citation2001)), Diagnostic Instrument for Mild Aphasia (DIMA, Satoer et al. (Citation2022)), category and letter fluency (Luteijn & Barelds, Citation2004; Schmand et al., Citation2008), Trail Making Test (TMT, Tombaugh (Citation2004)) and Hopkins Verbal Learning Test (HVLT, Benedict et al. (Citation1998)). See for a description of all tests. Due to aphasia severity (based on TT and clinical impressions by the SLP and/or frustration by the patient) some patients were not able to complete all tests. In that case priority was given to tests that were necessary for the intraoperative procedure. Additional information necessary for the intraoperative procedure could be collected by using tasks of DuLIP or screenings for language (Aphasia Bedside Check, ABC, Visch-Brink & El Hachioui (Citation2013)) or cognition (Montreal Cognitive Assessment, MOCA, Thissen et al. (Citation2010)).
Table 2. Neurolinguistic assessment
Based on the normative data from the manual of each individual (sub)test, z-scores were computed to compare performance of the patients to healthy controls. A clinical (mild) impairment is reflected by a z-score between -1.5 and -2.0; a pathological (severe) impairment is reflected by a z-score of ≤ -2.0. A change of 1.5 in z-score between pre- and postoperative testing is defined as clinically significant.
Results
Preoperatively, in A1, B2 and E5 the tumour showed large cystic and necrotic portions (see ). Tumour volume ranged from 37.19 to 81.75 cm3 preoperatively and from 0.00 to 16.11 cm3 postoperatively. The extent of resection ranged from 68 to 100%. Pathological examination of tumour tissue obtained during resection revealed glioblastoma in all patients except E5. A1 and B2 had histologically confirmed methylated promotor of O‐6‐methylguanine‐DNA methyltransferase (MGMT). A1, B2 and D4 had histologically confirmed isocitrate dehydrogenase wildtype (IDH-wildtype). Despite histologically confirmed glioblastoma after E5’s earlier resections, current histological analysis revealed anaplastic pleiomorf xanthoastrocytoma. A1, B2 and C3 underwent postoperative radiotherapy (A1 30 fraction doses of 2 Gy; B2 and C3 15 fraction doses of 2.67 Gy) and concomitant and adjuvant temozolomide. D4 received adjuvant lomustine. E5 received adjuvant dabrafenib and trametinib.
Adaptation of intraoperative tasks
For adequate interpretation during the intraoperative language monitoring, it is of crucial importance to select tasks and items that are preoperatively intact to ensure that the errors in the awake setting are not pre-existing but caused by DES or resection. In addition, the complexity of tasks can be adapted to preoperative level by adjusting stimuli according to different linguistic variables.
In word repetition, complexity level is influenced by word-length, the presence of consonant clusters and/or phonemic similarities (Gierut, Citation2007). As the repetition of 3-syllabic words from the DIMA in A1 or DuLIP Repetition (B2) was unimpaired, all 3-syllabic words with and without consonant clusters and phonemic similarities could be selected from DuLIP Repetition. As in C3, all DIMA Repetition subtests were impaired, repetition was not administered intraoperatively. As in D4 and E5, the repetition of 3-syllabic words appeared to be impaired at intraoperative baseline, 2-syllabic words with and without consonant clusters and phonemic similarities were selected from DuLIP Repetition.
Complexity level of object naming (black and white line drawings) is influenced by word frequency and age of acquisition (Brysbaert & Ellis, Citation2016). Due to impaired object naming in all patients attested with the BNT high-frequent, early acquired items were selected from DuLIP Object Naming.
Due to impaired verbal comprehension of longer sentences in all patients attested with the shortened TT semantic judgment of word pairs and words to categories, were presented via the dual input route, that is both visually and auditorily.
Neurolinguistic results
Below cases A1, B2, C3, D4 and E5 are presented. See for all neurolinguistic results.
Table 3. Pre- and postoperative neurolinguistic test-protocol (raw score/z-score).
A1 Left frontal lobe
Preoperatively, the spontaneous speech of A1 consisted of short sentences including hesitations and slow speech rate. Due to reduced verbal language comprehension in A1, questions had to be repeated during the interview before test administration. In general, verbal communication was limited. At test-level, A1 had preoperatively severely impaired scores in most language tests (TT: z = -5.74, BNT: z = -2.89, Category fluency: animals z = -3.20 and professions z = -3.30, Letter fluency: z = -2.70, DIMA Sentence completion: z = -4.14) and in subtests of the TMT for attention and executive functions (TMT-A: z = -3.50, TMT-B: z = -4.50, TMT-B/A: z = -3.10). Errors in the BNT consisted of no responses, paraphasias or neologisms were not produced. Mild impairments were observed in the total score of DIMA Repetition (z = -1.91) and DIMA Semantic odd-picture-out (z = -1.96). In DIMA Repetition subtests, 3-syllabic words and compounds were errorless but non-words and sentences contained some substitutions, omissions and hesitations (e.g. non-words: slanugari → slangugari; sentences: Iedere vrijdag eten we vreselijke vissticks/Every Friday we eat terrible fish fingers → Iedere vrijdag eten we vreselijke vv uh vissticks/Every Friday we eat terrible ff uh fish fingers). Due to aphasia severity, a test for verbal memory (direct recall, delayed recall and recognition) was not administered.
Intraoperatively, DES elicited no positive sites during repetition. During resection, performance of object naming, semantic judgment tasks, reading words aloud, sentence completion and spontaneous speech remained at the preoperative level. The resection boundary was reached when newly developed delayed responses, semantic paraphasias and perseverations were observed during object naming.
At seven weeks postoperatively, no language complaints were reported. At test-level, most tests recovered to intact scores compared to normative data (TT: z = -0.32, BNT: z = -0.07, Category fluency: animals: z = -0.60 and professions: z = -1.20, DIMA Repetition: z = 0.25, DIMA Semantic odd-picture-out: z = -0.69, DIMA Sentence completion: z = -0.63, TMT-A: z = -0.20). Letter fluency remained mildly impaired (z = -1.50). TMT-B improved significantly compared to preoperative level but remained severely impaired compared to normative data (z = -2.10). TMT-B/A remained severely impaired (TMT-B/A: z = -2.20). Overall, A1 improved on eight out of 11 administered tests.
B2 Left frontal lobe
Preoperatively, the spontaneous speech of B2 was non-fluent, effortful and sentences consisted of two or three words in combination with word finding difficulties and hesitations. Conversation about familiar topics was possible with help from the test-leader. However, B2 mostly did not manage to convey the message across. At test-level, B2 had severely impaired scores on most tests (TT: z = -13.60, BNT: z = -5.70, Category Fluency: animals z = -3.80 and professions z = -3.30, MOCA: z = -9.27). Errors in the BNT consisted of perseverations, semantic paraphasias (e.g helikopter/helicopter → vliegtuig/airplane) and circumlocutions. In case of the latter, the given descriptions were not always adequate (e.g slak/snail → op de grond/on the floor). DuLIP Repetition (shortened version) of 3-syllabic words was possible but three out of five sentences contained phonemic paraphasias, hesitations, and omissions (e.g. de koningin rookt een sigaret /the queen smokes a cigarette → rookt/smokes). Due to aphasia severity, letter fluency and tests for verbal memory, attention and executive functions were not administered.
Intraoperatively, DES triggered non-reproducible apraxia of speech (problems of initiation of speech and visual groping) and speech arrest in word repetition. During resection, performance of object naming, semantic judgment tasks, spontaneous speech and repetition remained at preoperative level. The resection boundary was reached when speech arrests occurred in spontaneous speech and in repetition.
At three months postoperatively, B2 reported no language complaints during the interview before test administration. At test-level, all tests remained severely impaired compared to normative data (TT: z = -6.75, Category fluency – animals: z = -3.20, Category fluency - professions: z = -2.90, ABC: 11/14, MOCA: z = -6.55), although a significant improvement compared to preoperative level was observed in the TT and the MOCA. Letter fluency improved slightly, as administration was now possible, although severely impaired compared to normative data (z = -2.50). Due to a different test-protocol, naming was screened with the ABC and resulted in two out of three items (low-frequency words) correct. Overall, B2 improved on three out of five administered tests.
C3 Left temporal lobe
Preoperatively, the spontaneous speech of C3 consisted of short sentences (telegraphic speech) with word-finding difficulties, neologisms, perseverations, semantic and phonemic paraphasias. At test-level, C3 had severely impaired scores in most language tests (TT: z = -15.08, BNT: z = -2.37, DIMA Repetition: z = -14.29, DIMA Semantic odd-picture-out: z = -5.77). Errors in the BNT consisted of neologisms (e.g. pelikaan/pelican → lebelberk), semantic and phonemic paraphasias. The DIMA Repetition subtests were intact for 3-syllabic words, but non-words contained substitutions (e.g. non-word: anato → inaro) and sentence repetition contained omissions (e.g. sentences: moeder roerde in de soep/mother stirs the soup → moeder is soep/mother is soup). DuLIP Semantic Sentence completion (z = -1.50) was mildly impaired when presented visually, whereas the auditory version of DIMA Sentence completion was not possible at all. Semantic DuLIP-tasks were feasible without time constraints (DuLIP Semantic odd-word-out: z > 0, DuLIP Semantic odd-picture-out: z > 0). Due to aphasia severity, letter fluency and tests for verbal memory, attention and executive functions were not administered.
Intraoperatively, DES elicited new semantic paraphasias and neologisms during object naming, which were not reproducible. During resection, performance of object naming, semantic judgment tasks, reading words aloud, sentence completion and spontaneous speech remained at the preoperative level. The resection boundary was reached when new neologisms and perseverations occurred during object naming.
At three months postoperatively, C3 reported no changes in language functioning during the interview before test administration. At test level, the TT and the BNT improved significantly compared to preoperative level. However, compared to normative data, the TT remained severely impaired (z = -12.05) and the BNT recovered to an intact performance (z = -0.62). DIMA Semantic odd-picture-out (z = -5.77) remained severely impaired. DuLIP Sentence Completion (z > -1.50) recovered to intact score. DuLIP Semantic odd-word-out (z > 0) remained intact. DIMA Repetition deteriorated further and could not be administered due to defective auditory input route. Overall, C3 improved on two out of six administered tests.
D4 Left temporo-parietal lobe
Preoperatively, the spontaneous speech of D4 was marked with word finding difficulties, stereotypes and incomplete sentences. Speech appeared to be occasionally mildly dysarthric. At test-level, D4 had severely impaired scores on most tests (TT: z = -5.23, BNT: z = -9.60, Category fluency: animals z = -3.20 and professions z = -3.30, DIMA Repetition: z = -7.29, DIMA Semantic odd-picture-out: z = -5.77, DIMA Sentence completion: z = -4.14, TMT-A: z = -3.50, TMT-B: z = -4.70, TMT-B/A: z = -3.40, HVLT recall: z = -5.21, HVLT delayed recall: z = -5.47, HVLT true positives: z = -2.00). Only HVLT false positives was intact (z = -0.33). Errors in the BNT consisted of semantic paraphasias, neologisms and circumlocutions. In DIMA Repetition 3-syllabic words were intact but compounds, non-words and sentences were severely impaired. Due to aphasia severity, letter fluency could not be administered.
Intraoperatively, DES elicited delayed reactions in repetition at cortical level and subcortical stimulation elicited light flashes. During resection, spontaneous speech, repetition and semantic judgment remained at preoperative level. Resection ended when the tumour was macroscopically completely removed in combination with slight deterioration of spontaneous speech.
One day postoperatively a short language screening, the ABC, was administered. Subsection Language Comprehension was intact (7/7), but in the subsection Language Production semantic paraphasias were produced in object naming and in reading aloud (5/7). Two weeks postoperatively D4 still reported word finding difficulties, but in general, speech production had improved. Due to a fall of the stairs and a bone flap infection, follow-up neurolinguistic assessment was not possible.
E5 Left temporo-parietal lobe
Preoperatively, the spontaneous speech of E5 was fluent despite its slow speech rate with semantic and phonemic paraphasias. Auditory comprehension was delayed. At test-level, E5 had severely impaired scores in most language tests (BNT: z = -12.80, Category fluency – animals: z = -3.60, Category fluency - professions: z = -3.80, Letter fluency: z = -3.80, DIMA Repetition: z = -14.83, DIMA Semantic odd-picture-out: z = -5.77). Errors in the BNT consisted of word finding difficulties, semantic paraphasias and circumlocutions (schaar/scissors → vanmiddag nog gebruikt/used this afternoon). In DIMA Repetition 3-syllabic words were nearly intact but compounds, non-words and sentences contained substitutions, phonemic paraphasias, self-corrections and omissions (sentences: de kinderen graven een put/the children dig a well → de kinderen een put/the children a well). DIMA Sentence completion (z = -0.63) was intact. Due to aphasia severity, the TT and tests for verbal memory, attention and executive functions were not administered.
Intraoperatively, no positive sites were identified during cortical and subcortical stimulation. During resection, spontaneous speech, object naming, repetition and semantic odd-picture-out remained at preoperative level. Resection ended when the tumour was macroscopically completely removed.
Two weeks and six months postoperatively, E5 reported improved spontaneous speech. E5 did not receive a follow-up neurolinguistic assessment.
Discussion
We aimed to examine the feasibility of awake surgery in patients with glioblastoma in eloquent language areas suffering from severe preoperative aphasia. Resection of glioblastoma using awake surgery was feasible in five patients, without perioperative complications. By using a patient-tailored intraoperative language monitoring, intraoperative language deterioration was distinguished from preoperative aphasia (Research question 1). Postoperatively, language was stable or even improved (Research question 2).
Language outcomes
Preoperatively, all patients had severe aphasia as measured with the TT, the BNT and the DIMA which is in accordance with other studies in which patients with faster growing tumours like glioblastoma, were more impaired than reported in low-grade glioma patients (Noll et al., Citation2015; Whittle et al., Citation1998). In the present study, cognitive tests could not be administered in some patients due to aphasia severity as these tests have heavy language demands. Non-verbal screening of cognition could be a solution for future research. When preoperative administration of cognitive tests was possible, severely impaired scores were found which is in line with other studies in high-grade glioma (Noll et al., Citation2015; van Kessel et al., Citation2017; van Kessel et al., Citation2019).
Intraoperatively, we used patient-tailored language tasks which were based on the preoperative extensive neurolinguistic examination on different input and output routes and an error analysis. As hypothesized, using such tasks we could intraoperatively make a reliable interpretation of further language deterioration and thereby detect the functional resection boundaries for language in all patients, which means that the resection was stopped when language deteriorated further compared to the preoperative aphasia. With our patient-tailored method, it seems that severely aphasic patients can be eligible for awake surgery.
Postoperatively, no further deterioration of aphasia severity occurred in any of the five patients. All patients reported improvement of language. As in brain tumour patients, the patients’ self-report is not always in accordance to proxy-report and to test performance (van der Linden et al., Citation2020), also an interview with a caregiver needs to be a part of standard practice. In our patients, language and other cognitive functions remained stable and even improved in most patients which is in line with our hypothesis. Most improvement was observed in A1 (eight out of eleven tests), followed by B2 (three out of five) and C3 (two out of six); an extensive postoperative examination in D4 and E5 was missing. Administration of DIMA Repetition in C3 was not possible postoperatively as due to a deficit in verbal comprehension. Earlier studies already demonstrated no negative effects of awake surgery on language functions in glioblastoma patients, although the inclusion of severely aphasic patients had never been reported so far (Altieri et al., Citation2019; Bonifazi et al., Citation2020; van Kessel et al., Citation2020). In other studies with low-grade glioma and high-grade glioma patients, persistent or new post-surgical language impairments were influenced by the presence of a preoperative aphasia (Finch & Copland, Citation2014; Ilmberger et al., Citation2008; McGirt et al., Citation2009). In our patients this did not appear to be the case.
Possible linguistic predictors of language outcomes
Although the postoperatively tested patients showed improvement on the TT, reflecting a decrease of aphasia severity, the extent of improvement varied across patients. A1 recovered to normal performance, B2 improved from a severe aphasia to a moderate aphasia and C3 remained severely impaired, despite a significant change in tests scores. It is possible that preoperative performance on word-finding, phonology, and/or semantics could have played a predictive role in the course of postoperative improvement. Ilmberger et al. (Citation2008) found that a preoperative subnormal naming performance appeared to be a prognostic factor for postoperative language deterioration after awake surgery in low-grade glioma and high-grade glioma. In all our patients, naming was impaired preoperatively. A1 and C3 improved to normal naming scores postoperatively contrasting with Ilmberger et al.’s (Citation2008) findings in which most patients had lower graded gliomas (WHO grade I-III). Currently, it is known that an extensive cortical and subcortical network is involved in word-retrieval processes, such as naming (Papagno et al., Citation2011). As our patients suffered from high-grade glioma (WHO grade IV), classically known to suppress cortical areas without infiltrating subcortical tracts, it is plausible that their postoperative recovery in naming performance is due to a release of mass-effect on cortico-subcortical structures.
It could also be speculated that an intact preoperative level in a phonology test (repetition) has predictive value for postoperative language outcome. in our patients preoperative phonological function attested with a repetition task was a predictor of postoperative language outcome. Both A1 and B2 had no preoperative severe impairment in repetition, whereas C3 was severely impaired in repetition which may explain these different paths of aphasia recovery. In stroke research, performance in phonological tests already appeared to be the strongest predictor of long-term outcome of aphasia (El Hachioui et al., Citation2013; Glize et al., Citation2017). Also, in low-grade glioma and high-grade glioma, Sierpowska et al. (Citation2017) showed that repetition during awake surgery was more sensitive than object naming to preserve the arcuate fasciculus and thereby preserving language. Despite the lack of recovery on the TT (significant improvement in z-score but still below the cut-off) in C3, it is noteworthy that naming recovered to normal performance and that the DuLIP semantic tasks remained almost intact. Hence, in this case it seemed of crucial importance to intraoperatively monitor the core of the lexical system, that is the semantic system to preserve or even improve postoperative language performance (Ellis & Young, Citation1996). When the semantic system could not be reached due to disturbances in verbal comprehension (a defective auditory input route) as in C3, written stimuli (orthographic input route) were needed to reach and thereby test the semantic system. Finally, C3 is the only patient that used neologisms in the pre- and intraoperative phase. This type of language error may be an indicator of worse language outcome as opposed to other types of paraphasias.
Possible clinical predictors of language outcome
Tumour location may also have influenced the linguistic profile and outcome. Our patients did not show a so-called location-to-function deficit, that is a language impairment specific to the linguistic functions that are commonly described in the literature for their tumour locations at cortico-subcortical level (De Witte et al., Citation2015). For example, the semantic odd-picture-out test from DuLIP could be applied in proximity of the posterior inferior temporal gyrus and/or the inferior fronto-occipital fasciculus. With the application of a location-to-function approach, specific linguistic tests may be more sensitive to elicit certain language errors (in this examples semantic paraphasias) indicating critical language areas. Preoperatively A1, B2 and C3 presented “Broca-like” telegraphic speech output, although this was only “classically” expected for A1 and B2, given the tumour location in the middle frontal gyrus near Broca’s area. All patients produced semantic paraphasias in spontaneous speech and/or naming tests which was expected for C3, D4 and E5 due to temporal tumours. It seems that all patients suffered from a broader and more mixed type of aphasia as seen after stroke than as typically described in low-grade glioma (Davie et al., Citation2009).
Tumour and other clinical characteristics may also have influenced language performance. Three of our patients (A1, B2, D4) had confirmed IDH-wildtype which is associated with more severe cognitive impairments (van Kessel et al., Citation2020; Wefel et al., Citation2016). Cystic and necrotic lesions have been described to be associated with postoperative improvement of psychomotor speed and visuospatial functioning (van Kessel et al., Citation2020). A1, B2 and E5 had a large cystic and necrotic portion in their preoperative tumour volume. Possibly this accounted for their improvement of language and cognitive performance at test-level (A1 and B2) or patient’s self-report during the interview (E5).
It is known that the glucocorticoide medication dexamethasone, which is often prescribed for brain tumour patients, effectively minimizes neurological symptoms (Palombi et al., Citation2018; Prado & Crowe, Citation2019). At the moment of preoperative neurolinguistic assessment, dexamethasone was only prescribed to C3. The amount of postoperative language improvement in C3 is thereby a result of surgery alone. Dexamethasone was prescribed a day preoperatively in A1 and B2. Their language improvement could therefore not only be a result of surgery but also due to a reduction of oedema by dexamethasone.
Two of our patients (A1 and B2) had a survival time of more than 34 months. Both of them showed postoperative improvement of their aphasia. Survival time in the other patients was 11 (C3), 15 months (D4) and 18 months (E5) for which D4 and E5 the tumour was recurrent. A negative influence of aphasia and cognitive impairment on survival was revealed in different studies (McGirt et al., Citation2009; Rahman et al., Citation2017; van Kessel et al., Citation2021). It could be the case that the postoperative language improvement in A1 and B2 contributed to a longer survival time as a result from a large tumour resection with a release of mass effect. Another explanation of longer survival of A1 and B2 could originate from their histologically confirmed methylated promotor of MGMT.
Limitations
Some limitations should be taken into account when interpreting our results. We described only five patients which is a very small group to draw solid conclusions from. Postoperative assessment was conducted at different time points. In addition, A1 and B2 were tested after finishing their radiotherapy which may have influenced the results when measuring the effect of surgery on language functions. Despite this, both patients showed improvement of language functions. D4 and E5 had recurrent glioblastoma and had already underwent surgery with general anaesthesia twice and had received extensive chemo-radiation which may have influenced the preoperative scores before awake surgery. Due to a typical postoperative decline in the acute phase it is recommended that assessment takes place after finishing radiotherapy which is approximately three months postoperatively.
Reading assessment is necessary to investigate whether the orthographic input route can be used to present language tasks during surgery. In C3 auditory comprehension was limited, but not written comprehension. A dual input route makes it possible to reach the semantic system. In our patients, reading assessment was based on clinical interpretation of scores on various tests. It is advised to administer reading tests in future formal examinations to ascertain the intraoperative use of the orthographic input route.
Future directions
Findings in these case series suggest, in line with Gerritsen et al. (Citation2022), that awake surgery in glioblastoma patients is safe and feasible, but our study suggests it is even possible when patients have preoperative severe aphasia. A prospective multicentre randomized clinical trial (SAFE-trial NL7589 by Gerritsen (et al. (Citation2020)) is ongoing to assess the added value of awake surgery in glioblastoma. High expertise of a multidisciplinary awake team is mandatory including a specialized SLP. More standardized intraoperative tasks for severe aphasia are needed for this population. Our team is working on a revised version of DuLIP (De Witte et al., Citation2015) with tasks designed especially for patients with severe aphasia.
Clinical recommendations
For adequate intraoperative monitoring of severely aphasic glioblastoma patients, extensive preoperative neurolinguistic examination is necessary with special attention for repetition and semantic tasks in addition to the traditional object naming test. All input and output routes at different linguistic levels need to be thoroughly examined according to a language processing model including a language error analysis. This way, intraoperative tasks can be selected adequately and adapted to the preoperative language level. By doing so, the SLP can focus on the intact linguistic levels, thereby facilitating reliable interpretation of further language deterioration during DES and resection. When new paraphasias occur, resection boundaries for language are reached. Consequently, a maximal safe resection can be achieved with language preservation, even in glioblastoma patients with severe aphasia.
Conclusion
We described for the first time the feasibility of awake surgery in patients with glioblastoma in eloquent language areas suffering from preoperative severe aphasia. For adequate intraoperative monitoring of severely aphasic glioblastoma patients, a preoperative extensive neurolinguistic examination and a subsequent adaptation of intraoperative tasks is necessary. In this way functional boundaries for language can be detected even in preoperatively severely aphasic patients resulting in stable or improved language outcome.
Abbreviations
ABC = Aphasia Bedside Check BNT = Boston Naming Test DES = direct electrical stimulation DIMA = Diagnostic Instrument for Mild Aphasia DuLIP = Dutch Linguistic Intraoperative Protocol HVLT = Hopkins Verbal Learning Test IDH = molecular marker isocitrate dehydrogenase MGMT = molecular marker O‐6‐methylguanine‐DNA methyltransferase MOCA = Montreal Cognitive Assessment SLP = speech and language pathologist TMT = Trail Making Test TT = shortened Token Test
Acknowledgments
We thank Markus Klimek, Ismail Eralp, Stephanie Lequin-Van Duijn, Wim van Alphen, anaesthesia and surgeon’s assistants of Erasmus MC and HMC for their intraoperative contribution and Evy Visch-Brink for her contribution to the newly developed intraoperative language tasks. Marike Donders-Kamphuis was funded by a grant from the Research Fund of Haaglanden Medisch Centrum (Wetenschapsbeurs 2021).
Supplemental Material
Download MS Word (289.2 KB)Supplemental data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/02687038.2022.2137773.
Disclosure statement
No potential competing interest was reported by the authors
References
- Altieri, R., Raimondo, S., Tiddia, C., Sammarco, D., Cofano, F., Zeppa, P., Monticelli, M., Melcarne, A., Junemann, C., Zenga, F., Savastano, R., Garbossa, D., Certo, F., & Barbagallo, G. (2019). Glioma surgery: From preservation of motor skills to conservation of cognitive functions. Journal of Clinical Neuroscience, 70, 55–60. 10.1016/j.jocn.2019.08.091
- Benedict, R. H. B., Schretlen, D., Groninger, L., & Brandt, J. (1998). Hopkins Verbal Learning Test–Revised: Normative data and analysis of inter-form and test-retest reliability. The Clinical Neuropsychologist, 12(1), 43–55.
- Bonifazi, S., Passamonti, C., Vecchioni, S., Trignani, R., Martorano, P. P., Durazzi, V., Lattanzi, S., Mancini, F., & Ricciuti, R. A. (2020). Cognitive and linguistic outcomes after awake craniotomy in patients with high-grade gliomas. Clinical Neurology and Neurosurgery, 198, 106089. 10.1016/j.clineuro.2020.106089
- Brysbaert, M., & Ellis, A. W. (2016). Aphasia and age of acquisition: are early-learned words more resilient? Aphasiology, 30(11), 1240–1263.
- Dallabona, M., Sarubbo, S., Merler, S., Corsini, F., Pulcrano, G., Rozzanigo, U., Barbareschi, M., & Chioffi, F. (2017). Impact of mass effect, tumor location, age, and surgery on the cognitive outcome of patients with high-grade gliomas: a longitudinal study. Neurooncol Pract, 4(4), 229–240. 10.1093/nop/npw030
- Davie, G. L., Hutcheson, K. A., Barringer, D. A., Weinberg, J. S., & Lewin, J. S. (2009). Aphasia in patients after brain tumour resection. Aphasiology, 23(9), 1196–1206.
- De Renzi, E., & Faglioni, P. (1978). Normative data and screening power of a shortened version of the Token Test. Cortex, 14(1), 41–49. 10.1016/s0010-9452(78)80006-9
- De Witte, E., & Marien, P. (2013). The neurolinguistic approach to awake surgery reviewed. Clinical Neurology and Neurosurgery, 115(2), 127–145. 10.1016/j.clineuro.2012.09.015
- De Witte, E., Satoer, D., Robert, E., Colle, H., Verheyen, S., Visch-Brink, E., & Marien, P. (2015). The Dutch Linguistic Intraoperative Protocol: a valid linguistic approach to awake brain surgery. Brain and Language, 140, 35–48. 10.1016/j.bandl.2014.10.011
- De Witt Hamer, P. C., Robles, S. G., Zwinderman, A. H., Duffau, H., & Berger, M. S. (2012). Impact of intraoperative stimulation brain mapping on glioma surgery outcome: a meta-analysis. Journal of Clinical Oncology, 30(20), 2559–2565. 10.1200/JCO.2011.38.4818
- Dziedzic, T., & Bernstein, M. (2014). Awake craniotomy for brain tumor: indications, technique and benefits. Expert Review of Neurotherapeutics, 14(12), 1405–1415. 10.1586/14737175.2014.979793
- El Hachioui, H., Lingsma, H. F., van de Sandt-Koenderman, M. W., Dippel, D. W., Koudstaal, P. J., & Visch-Brink, E. G. (2013). Long-term prognosis of aphasia after stroke. Journal of Neurology, Neurosurgery and Psychiatry, 84(3), 310–315. 10.1136/jnnp-2012-302596
- Ellis, A. W., & Young, A. W. (1996). Human Cognitive Neuropsychology: A Textbook with Readings. Psychology Press.
- Finch, E., & Copland, D. A. (2014). Language outcomes following neurosurgery for brain tumours: a systematic review. NeuroRehabilitation, 34(3), 499–514. 10.3233/NRE-141053
- Gerritsen, J. K. W., Arends, L., Klimek, M., Dirven, C. M. F., & Vincent, A. J. E. (2019). Impact of intraoperative stimulation mapping on high-grade glioma surgery outcome: a meta-analysis. Acta Neurochirurgica, 161(1), 99–107. 10.1007/s00701-018-3732-4
- Gerritsen, J. K. W., Klimek, M., Dirven, C. M. F., Hoop, E. O., Wagemakers, M., Rutten, G. J. M., Kloet, A., Hallaert, G. G., & Vincent, A. (2020). The SAFE-trial: Safe surgery for glioblastoma multiforme: Awake craniotomy versus surgery under general anesthesia. Study protocol for a multicenter prospective randomized controlled trial. Contemporary Clinical Trials, 88, 105876. 10.1016/j.cct.2019.105876
- Gerritsen, J. K. W., Zwarthoed, R. H., Kilgallon, J. L., Nawabi, N. L., Jessurun, C. A. C., Versyck, G., Pruijn, K. P., Fisher, F. L., Lariviere, E., Solie, L., Mekary, R. A., Satoer, D. D., Schouten, J. W., Bos, E. M., Kloet, A., Nandoe Tewarie, R., Smith, T. R., Dirven, C. M. F., De Vleeschouwer, S., Broekman, M. L. D., & Vincent, A. (2022). Effect of awake craniotomy in glioblastoma in eloquent areas (GLIOMAP): a propensity score-matched analysis of an international, multicentre, cohort study. Lancet Oncology, 23(6), 802–817. 10.1016/S1470-2045(22)00213-3
- Gierut, J. A. (2007). Phonological complexity and language learnability. American Journal of Speech-Language Pathology, 16(1), 6–17. 10.1044/1058-0360(2007/003)
- Glize, B., Villain, M., Richert, L., Vellay, M., de Gabory, I., Mazaux, J. M., Dehail, P., Sibon, I., Laganaro, M., & Joseph, P. A. (2017). Language features in the acute phase of poststroke severe aphasia could predict the outcome. European Journal of Physical and Rehabilitation Medicine, 53(2), 249–255. 10.23736/S1973-9087.16.04255-6
- Habets, E. J., Kloet, A., Walchenbach, R., Vecht, C. J., Klein, M., & Taphoorn, M. J. (2014). Tumour and surgery effects on cognitive functioning in high-grade glioma patients. Acta Neurochirurgica, 156(8), 1451–1459. 10.1007/s00701-014-2115-8
- IJzerman-Korevaar, M., Snijders, T. J., de Graeff, A., Teunissen, S., & de Vos, F. Y. F. (2018). Prevalence of symptoms in glioma patients throughout the disease trajectory: a systematic review. Journal of Neuro-Oncology, 140(3), 485–496. 10.1007/s11060-018-03015-9
- Ilmberger, J., Ruge, M., Kreth, F. W., Briegel, J., Reulen, H. J., & Tonn, J. C. (2008). Intraoperative mapping of language functions: a longitudinal neurolinguistic analysis. Journal of Neurosurgery, 109(4), 583–592. 10.3171/JNS/2008/109/10/0583
- Kaplan, E., Goodglass, H., & Weintraub, S. (2001). Boston naming test. Pro-ed.
- Li, Y. C., Chiu, H. Y., Lin, Y. J., Chen, K. T., Hsu, P. W., Huang, Y. C., Chen, P. Y., & Wei, K. C. (2021). The Merits of Awake Craniotomy for Glioblastoma in the Left Hemispheric Eloquent Area: One Institution Experience. Clinical Neurology and Neurosurgery, 200, 106343. 10.1016/j.clineuro.2020.106343
- Luteijn, F., & Barelds, D. P. H. (2004). GIT2: Groninger Intelligentie Test 2. Harcourt Test Publishers.
- McGirt, M. J., Mukherjee, D., Chaichana, K. L., Than, K. D., Weingart, J. D., & Quinones-Hinojosa, A. (2009). Association of surgically acquired motor and language deficits on overall survival after resection of glioblastoma multiforme. Neurosurgery, 65(3), 463–469; discussion 469-470. 10.1227/01.NEU.0000349763.42238.E9
- Noll, K. R., Sullaway, C., Ziu, M., Weinberg, J. S., & Wefel, J. S. (2015). Relationships between tumor grade and neurocognitive functioning in patients with glioma of the left temporal lobe prior to surgical resection. Neuro-Oncology, 17(4), 580–587. 10.1093/neuonc/nou233
- Palombi, L., Marchetti, P., Salvati, M., Osti, M. F., Frati, L., & Frati, A. (2018). Interventions to Reduce Neurological Symptoms in Patients with GBM Receiving Radiotherapy: From Theory to Clinical Practice. Anticancer Research, 38(4), 2423–2427. 10.21873/anticanres.12494
- Papagno, C., Gallucci, M., Casarotti, A., Castellano, A., Falini, A., Fava, E., Giussani, C., Carrabba, G., Bello, L., & Caramazza, A. (2011). Connectivity constraints on cortical reorganization of neural circuits involved in object naming. Neuroimage, 55(3), 1306–1313. 10.1016/j.neuroimage.2011.01.005
- Prado, C. E., & Crowe, S. F. (2019). Corticosteroids and Cognition: A Meta-Analysis. Neuropsychology Review, 29(3), 288–312. 10.1007/s11065-019-09405-8
- Rahman, M., Abbatematteo, J., De Leo, E. K., Kubilis, P. S., Vaziri, S., Bova, F., Sayour, E., Mitchell, D., & Quinones-Hinojosa, A. (2017). The effects of new or worsened postoperative neurological deficits on survival of patients with glioblastoma. Journal of Neurosurgery, 127(1), 123–131. 10.3171/2016.7.JNS16396
- Rofes, A., Mandonnet, E., Godden, J., Baron, M. H., Colle, H., Darlix, A., de Aguiar, V., Duffau, H., Herbet, G., Klein, M., Lubrano, V., Martino, J., Mathew, R., Miceli, G., Moritz-Gasser, S., Pallud, J., Papagno, C., Rech, F., Robert, E., Rutten, G. J., Santarius, T., Satoer, D., Sierpowska, J., Smits, A., Skrap, M., Spena, G., Visch, E., De Witte, E., Zetterling, M., & Wager, M. (2017). Survey on current cognitive practices within the European Low-Grade Glioma Network: towards a European assessment protocol. Acta Neurochirurgica, 159(7), 1167–1178. 10.1007/s00701-017-3192-2
- Rofes, A., & Miceli, G. (2014). Language mapping with verbs and sentences in awake surgery: a review. Neuropsychology Review, 24(2), 185–199. 10.1007/s11065-014-9258-5
- Satoer, D., De Witte, E., Bulte, B., Bastiaanse, R., Smits, M., Vincent, A., Marien, P., & Visch-Brink, E. (2022). Dutch Diagnostic Instrument for Mild Aphasia (DIMA): standardisation and a first clinical application in two brain tumour patients. Clinical Linguistics & Phonetics, 1–25. 10.1080/02699206.2021.1992797
- Schmand, B., Groenink, S. C., & van den Dungen, M. (2008). [Letter fluency: psychometric properties and Dutch normative data] Letterfluency: psychometrische eigenschappen en Nederlandse normen. Tijdschrift voor Gerontologie en Geriatrie, 39(2), 64–76. 10.1007/BF03078128
- Sierpowska, J., Gabarros, A., Fernandez-Coello, A., Camins, A., Castaner, S., Juncadella, M., Moris, J., & Rodriguez-Fornells, A. (2017). Words are not enough: nonword repetition as an indicator of arcuate fasciculus integrity during brain tumor resection. Journal of Neurosurgery, 126(2), 435–445. 10.3171/2016.2.JNS151592
- Sierpowska, J., Rofes, A., Dahlslatt, K., Mandonnet, E., Ter Laan, M., Polczynska, M., Hamer, P. W., Halaj, M., Spena, G., Meling, T. R., Motomura, K., Reyes, A. F., Campos, A. R., Robe, P. A., Zigiotto, L., Sarubbo, S., Freyschlag, C. F., Broen, M. P. G., Stranjalis, G., Papadopoulos, K., Liouta, E., Rutten, G. J., Viegas, C. P., Silvestre, A., Perrote, F., Brochero, N., Caceres, C., Zdun-Ryzewska, A., Kloc, W., Satoer, D., Dragoy, O., Hendriks, M. P. H., Alvarez-Carriles, J. C., & Piai, V. (2022). The Aftercare Survey: Assessment and intervention practices after brain tumor surgery in Europe. Neurooncol Pract, 9(4), 328–337. 10.1093/nop/npac029
- Sinha, R., Stephenson, J. M., & Price, S. J. (2020). A systematic review of cognitive function in patients with glioblastoma undergoing surgery. Neurooncol Pract, 7(2), 131–142. 10.1093/nop/npz018
- Stupp, R., Mason, W. P., van den Bent, M. J., Weller, M., Fisher, B., Taphoorn, M. J., Belanger, K., Brandes, A. A., Marosi, C., Bogdahn, U., Curschmann, J., Janzer, R. C., Ludwin, S. K., Gorlia, T., Allgeier, A., Lacombe, D., Cairncross, J. G., Eisenhauer, E., Mirimanoff, R. O., European Organisation for, R., Treatment of Cancer Brain, T., Radiotherapy, G, & National Cancer Institute of Canada Clinical Trials, G. (2005). Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. New England Journal of Medicine, 352(10), 987–996. 10.1056/NEJMoa043330
- Tandon, P., Mahapatra, A. K., & Khosla, A. (1993). Operations on gliomas involving speech centres. Acta Neurochirurgica. Supplementum, 56, 67–71. 10.1007/978-3-7091-9239-9_11
- Thissen, A., Van Bergen, F., De Jonghe, J. F. M., Kessels, R. P. C., & Dautzenberg, P. L. J. (2010). Bruikbaarheid en validiteit van de Nederlandse versie van de Montreal Cognitive Assessment (MoCA-D) bij het diagnosticeren van Mild Cognitive Impairment. Tijdschrift voor Gerontologie en Geriatrie, 41(6), 231–240.
- Tombaugh, T. N. (2004). Trail Making Test A and B: normative data stratified by age and education. Archives of Clinical Neuropsychology, 19(2), 203–214. 10.1016/S0887-6177(03)00039-8
- van der Linden, S. D., Gehring, K., De Baene, W., Emons, W. H. M., Rutten, G. M., & Sitskoorn, M. M. (2020). Assessment of Executive Functioning in Patients with Meningioma and Low-Grade Glioma: A Comparison of Self-Report, Proxy-Report, and Test Performance. Journal of the International Neuropsychological Society, 26(2), 187–196. 10.1017/S1355617719001164
- van Kessel, E., Baumfalk, A. E., van Zandvoort, M. J. E., Robe, P. A., & Snijders, T. J. (2017). Tumor-related neurocognitive dysfunction in patients with diffuse glioma: a systematic review of neurocognitive functioning prior to anti-tumor treatment. Journal of Neuro-Oncology, 134(1), 9–18. 10.1007/s11060-017-2503-z
- van Kessel, E., Emons, M. A. C., Wajer, I. H., van Baarsen, K. M., Broekman, M. L., Robe, P. A., Snijders, T. J., & Van Zandvoort, M. J. E. (2019). Tumor-related neurocognitive dysfunction in patients with diffuse glioma: a retrospective cohort study prior to antitumor treatment. Neurooncol Pract, 6(6), 463–472. 10.1093/nop/npz008
- van Kessel, E., Huenges Wajer, I. M. C., Ruis, C., Seute, T., Fonville, S., De Vos, F., Verhoeff, J. J. C., Robe, P. A., van Zandvoort, M. J. E., & Snijders, T. J. (2021). Cognitive impairments are independently associated with shorter survival in diffuse glioma patients. Journal of Neurology, 268(4), 1434–1442. 10.1007/s00415-020-10303-w
- van Kessel, E., Snijders, T. J., Baumfalk, A. E., Ruis, C., van Baarsen, K. M., Broekman, M. L., van Zandvoort, M. J. E., & Robe, P. A. (2020). Neurocognitive changes after awake surgery in glioma patients: a retrospective cohort study. Journal of Neuro-Oncology, 146(1), 97–109. 10.1007/s11060-019-03341-6
- Visch-Brink, E., & El Hachioui, H. (2013). Afasie Bedside Check: Handleiding. Rotterdam: ErasmusMC.
- Wefel, J. S., Noll, K. R., Rao, G., & Cahill, D. P. (2016). Neurocognitive function varies by IDH1 genetic mutation status in patients with malignant glioma prior to surgical resection. Neuro-Oncology, 18(12), 1656–1663. 10.1093/neuonc/now165
- Whittle, I. R., Pringle, A. M., & Taylor, R. (1998). Effects of resective surgery for left-sided intracranial tumours on language function: a prospective study. Lancet, 351(9108), 1014–1018. 10.1016/S0140-6736(97)08295-0
- Yushkevich, P. A., Piven, J., Hazlett, H. C., Smith, R. G., Ho, S., Gee, J. C., & Gerig, G. (2006). User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage, 31(3), 1116–1128. 10.1016/j.neuroimage.2006.01.015
- Zigiotto, L., Annicchiarico, L., Corsini, F., Vitali, L., Falchi, R., Dalpiaz, C., Rozzanigo, U., Barbareschi, M., Avesani, P., Papagno, C., Duffau, H., Chioffi, F., & Sarubbo, S. (2020). Effects of supra-total resection in neurocognitive and oncological outcome of high-grade gliomas comparing asleep and awake surgery. Journal of Neuro-Oncology, 148(1), 97–108. 10.1007/s11060-020-03494-9

