Abstract
Objective
This post-authorization safety study (EU PAS Register Number: EUPAS16088) was designed to compare the incidence of cancer outcomes in patients treated with mirabegron versus antimuscarinic medications.
Methods
Cohorts of mirabegron initiators during 2012–2018 were propensity-score matched to antimuscarinic medication initiators within real-world data sources (Danish National Registers, Swedish National Registers, Clinical Practice Research Datalink [UK], Optum [US], and Humana [US]). Incident cancer cases were identified during follow-up from direct linkage to cancer registers or validated through medical record review or through physician questionnaires. Comparisons of sex-specific composite cancer outcomes (cancer of the lung/bronchus, colon/rectum, melanoma of skin, urinary bladder, non-Hodgkin lymphoma, kidney/renal pelvis, pancreas, prostate in men and breast and uterus in women) were made overall and for person-time in the first year and after the first year following start of treatment, for all ages and for the subgroup ≥65 years.
Results
Among the 80,637 mirabegron initiators matched to 169,885 antimuscarinic medication initiators, 68% were at least 65 years of age and 66% were women. Over 5000 incident cancer cases were observed overall. Incidence rates were higher for men than women for composite and individual cancer outcomes. The pooled fixed effects hazard ratios for composite cancer outcomes (all ages) were 1.05 (95% confidence interval [CI]: 0.98–1.14) for women and 1.06 (95% CI: 0.98–1.14) for men. Results were similar in persons ≥65 years.
Conclusions
The results suggest no association between mirabegron use and risk of cancer, compared with antimuscarinic medications, in either men or women. Registration: EU PAS Register Number: EUPAS16088
Introduction
Overactive bladder (OAB) is a syndrome defined by the International Continence Society as “urinary urgency, usually with urinary frequency and nocturia, with or without urgency urinary incontinence”Citation1–3, if there is no proven infection or other obvious pathologyCitation4. A wide variety of approaches are used in the management of OAB. These include treating any underlying reversible medical conditions contributing to the syndrome and behavioral interventions aimed at re-establishing normal voiding intervals and continence (such as bladder training, managing fluid intake, pelvic floor muscle training, or weight control)Citation5. Pharmacologic agents and more invasive options, including surgery, are also availableCitation6.
For many years, antimuscarinic medications have been used to treat OAB symptomsCitation7. In 2012, the use of mirabegron was approved by the United States Food and Drug Administration (FDA) and the European Medicines Agency (EMA). Mirabegron is a β3-adrenergic receptor agonist that represents an alternative to antimuscarinics for treatment of OABCitation8. During the clinical development program for mirabegron, a numerical imbalance was observed in the number of neoplasms (malignant, benign, or unspecified) among patients randomized to mirabegron 100 mg (11 of 820; 1.3%) compared with those randomized to mirabegron 50 mg (1 of 812; 0.1%) or to tolterodine 4 mg (4 of 812; 0.5%) in a 52-week double-blind studyCitation9. In addition, in one of the six 12-week phase 2/3 randomized double-blind mirabegron OAB studies, serious adverse events were observed within the system organ class of neoplasms (benign, malignant, and unspecified [i.e. cysts and polyps]). These were numerically higher in the mirabegron 50 mg (3 of 442; 0.7%) and mirabegron 100 mg (2 of 433; 0.5%) groups versus the placebo group (1 of 453; 0.2%)Citation10, albeit without reaching statistical significance. The numerical imbalance was not observed in the remaining five phase 2/3 studies of the same 12-week durationCitation11–15. A pathophysiological mechanism to explain these observed numerical imbalances was not clear. At the time of approval, the FDA issued a post-marketing requirement (PMR) to evaluate cancer risks associated with mirabegron use, and a post-authorization safety study (PASS) was designed to address this concern. This manuscript reports on results from the PASS.
Materials and methods
Study population
The study population was formed using data sources from the US and Europe, which were the regions where mirabegron was initially approved. Data from patients covered in five electronic healthcare databases/national health registers in four countries were used for this study, which was a collaborative effort by research partners from the University of Southern Denmark (SDU, Danish National Registers); Centre for Pharmacoepidemiology, Karolinska Institutet (KI, Swedish National Registers); RTI Health Solutions (RTI-HS, Spain and USA), Clinical Practice Research Datalink [CPRD], UK); Optum (Optum Research Database [ORD], USA); and Humana Healthcare Research (HHR, Humana Database, USA). These data sources and research partners were selected based on their size, ability to provide the information needed for this study (e.g. available prescription or dispensing and hospitalization information, linkage to cancer registries or ability to conduct case validation), the inclusion of mirabegron in their formularies, previous participation in studies requested by regulatory authorities, and interest from each institution in the scientific question.
The CPRD population was analyzed as two groups: CPRD-linked (including data from the subset of UK general practices that sent data to CPRD and facilitate linkage to hospital, mortality, or cancer registry data) and CPRD-unlinked (data from UK general practices that sent data to CPRD but do not allow linkage to other data sources). Each research partner followed the same core protocol and statistical analysis plan, although operational details varied due to the specifics of the different data environments; therefore, site-specific protocols were developed. Within each research site, standard operating procedures and various quality control measures were used to guide the conduct of the study.Citation16,Citation17
Study design
The multi-site cohort study (EU PAS Register Number: EUPAS16088) included patients exposed to mirabegron or antimuscarinic medications (darifenacin, fesoterodine, oxybutynin, solifenacin, tolterodine, and trospium) to treat OAB. These 6 antimuscarinic medications were selected as they were globally available at the time of study design. The study period was from 1 October 2012 through 30 September 2018, although this differed between the data sources. Exposure was based on prescription or dispensing data, and only new users were included. The Danish National Registers, Swedish National Registers, ORD and HHR recorded prescriptions dispensed at a pharmacy, and the CPRD recorded issued prescriptions; “prescriptions” is used in this manuscript to refer to prescriptions issued or dispensed, as appropriate for each data source. A new user could be either a naïve new user, defined as a patient with a new prescription of a medication for treatment of OAB (mirabegron or antimuscarinic medication) without any OAB medication prescription during the 12-month period prior to a new prescription (i.e. baseline period), or a non-naïve new user, defined as a patient with a new prescription of an OAB medication who had a prescription for some other antimuscarinic medication during the 12-month baseline period. Each mirabegron user was matched with up to four antimuscarinic medication users by propensity scores (PS).
Inclusion/exclusion criteria
The individuals were required to meet all three of the following inclusion criteria to enter the study: a recorded prescription (defined as the index prescription) for mirabegron or tablet form of an antimuscarinic medication indicated for treatment of OAB (darifenacin, fesoterodine, oxybutynin, solifenacin, tolterodine, or trospium), with no prescription for that specific medication in the prior 12 months; age ≥18 years; and with ≥12 months of continuous enrollment in the data source before the index prescription of mirabegron or antimuscarinic medication. The 12-month baseline period provided medical and prescription history data and the ability to implement an operational definition of new use.
The main exclusion criterion was having at least one diagnosis code or evidence of any cancer (including in-situ cancers) other than non-melanoma skin cancer (NMSC). Evidence of cancer varied between data sources, and could include codes for history of cancer, mastectomy, hysterectomy, chemotherapy, or other cancer-related therapies. Patients with a prescription for mirabegron in all available observed data prior to the index prescription of mirabegron or an antimuscarinic medication were also excluded, but not those with a prescription for an antimuscarinic medication.
Exposure definition
Exposure to the study drugs was assessed using the prescription information recorded in each database. Starting with the first day after the date of the index prescription of mirabegron, all subsequent person-time was classified as mirabegron-exposed until the end of follow-up. Similarly, starting with the first day after the date of the index prescription of an antimuscarinic medication (or multiple antimuscarinic medications), all subsequent person-time was classified as antimuscarinic-exposed until the end of follow-up or a recorded prescription for mirabegron. If there was overlap between antimuscarinic medication days’ supply and the initiation of mirabegron, person-time assigned to the antimuscarinic medication was truncated as of the date of mirabegron prescription. If a patient was initially matched as an antimuscarinic medication new user and subsequently switched to or added mirabegron, all subsequent time was classified into mirabegron-exposed person-time, and if a patient was prescribed mirabegron and antimuscarinic medication on the same day, all subsequent person-time was categorized as mirabegron-exposed.
Cumulative dose definition
For each patient, cumulative dose was calculated for mirabegron and for the most frequently observed comparator antimuscarinic medication within each database. Cumulative dose was calculated by summing the total number of days’ supply multiplied by the strength of the tablets for all observed strengths separately for each drug, and cumulative dose was accrued during each day of follow-up. In data sources that directly capture days’ supply (i.e. ORD, HHR), the total number of days’ supply was calculated simply as the sum of days’ supply for all prescription during the post-index period. The start date was defined as the day after the index prescription date. This was calculated separately for matched cohorts of mirabegron and antimuscarinic medications. In data sources without days’ supply information (i.e. Swedish and Danish National Registers, CPRD), estimated days’ supply was determined using site-specific methods.
Outcome assessment
The cancer cases used for the analyses were identified either from direct linkage to cancer registers (Danish National Registers, Swedish National Registers, CPRD-linked), from medical chart adjudication (ORD, Humana Database), or electronic identification confirmed by questionnaires sent to physicians (CPRD-unlinked). Potential cases were identified using one of the following coding schemes for diagnoses, depending on the data source: International Classification of Diseases, 9th Revision (ICD-9)Citation18, International Classification of Diseases, 10th Revision (ICD-10)Citation19, ICD-9/ICD-10-Clinical Modification (CM)Citation20, ICD-Oncology (ICD-O)Citation21, or Read codes. Carcinoma in situ was not considered as an outcome.
Composite cancer endpoint
The primary endpoints were sex-specific composite cancer outcomes, defined as the first occurrence of any cancer of the lung/bronchus, colon/rectum, melanoma of skin, urinary bladder, non-Hodgkin’s lymphoma, kidney/renal pelvis, and pancreas for both men and women, and in addition, prostate cancer (for men), as well as breast and uterine cancer (for women). This range of cancers was chosen for assessment in this study due to the broad nature of the neoplasm signals detected in the clinical development programCitation9,Citation10. The individual cancers were selected from the ten most commonly occurring malignancies in the US (excluding NMSC)Citation22. This list largely overlaps with lists from other regions in the world, e.g. the five most frequently occurring types of cancer in the EU are colorectal cancer; trachea, lung/cancer; breast cancer; and prostate cancerCitation23.
Individual cancer-specific endpoint
For each of the ten cancer types, only the first cancer event identified after index date, and on or before the last day of follow-up, was counted in the individual cancer count. For example, once a patient met the breast cancer endpoint definition, that patient was censored from consideration for other individual cancer endpoints. For each individual cancer outcome, patients were excluded if they had prior evidence of a condition or procedure that made them not-at-risk for a particular cancer. For example, a woman who had a hysterectomy prior to the index date was excluded from the cohort for the uterine cancer analysis. However, if a woman had a hysterectomy during follow-up (i.e. after the index date, but prior to having a uterine cancer diagnosis code), she was censored from the uterine cancer outcome analysis on the date of the hysterectomy but was not censored for analyses of other cancer outcomes.
Follow-up
Follow-up of eligible patients started on the day after the index prescription for mirabegron or antimuscarinic medication. Two cohorts of person-time were defined following initiation of treatment: the person-time among new users of mirabegron and the person-time among new users of antimuscarinic medications.
Follow-up for the composite and individual cancer endpoints ended at the earliest of the following dates: end of the study period; last date of data with validated cancer outcomes within each of the data sources that could be linked to cancer registries; disenrollment from the data source (e.g. emigration, disenrollment from health plan, death); first observed occurrence of data source-specific criteria of any cancer other than NMSC, including the ten study cancers; and for individual cancer endpoints only, the date of a condition (e.g. bilateral mastectomy or hysterectomy) that made the patient not-at-risk for the specific cancer. In addition, for patients in the antimuscarinic medications cohort, this included the date for prescription of a non-tablet type of antimuscarinic medication (due to the difficulty assigning exposure-time during use of syrups, patches, gels, or intravesical medications).
Statistical methods
Data were analyzed separately within each of the five study populations and all coding was done independently by each research partner, with collaboration as needed. For the primary analysis of study endpoints, a meta-analysis was conducted using results pooled from all study populations.
Within each study population, PS were estimated by modeling the probability of treatment with mirabegron compared with treatment with any study antimuscarinic medication, conditional on the baseline covariates. A unique year-specific PS was calculated for each first initiation that met the study inclusion criteria using the relevant baseline variables for the initiation in that calendar year. The PS models included pre-specified variables common to all databases, including demographics (age/age group and sex), known risk factors for developing the individual cancers (e.g. use of hormone replacement therapy, inflammatory bowel disease, family history of cancers), and expected predictors of mirabegron versus antimuscarinic medication exposure (e.g. prior use of individual antimuscarinic medications). Database-specific pre-specified variables (e.g. geographic areas, length of enrollment in the health plan) and empirically-identified variables (e.g. most frequent ICD diagnosis codes, procedures, and medications prescribed/dispensed) were also included. The database-common pre-specified variables were forced into the PS models. The database-specific variables (pre-specified and empirically-identified) were considered for entry into the PS model through the stepwise automatic variable selection procedure. Variables with a p-value ≤ 0.10 were entered and remained in the model if the p-value was ≤ 0.30. Several checks were conducted for the variable selection process, including checking for correlations among pre-defined covariates and the empirically defined variables. Univariate statistics of the distributions of the PS were reviewed for potential coding errors and outliers.
Mirabegron initiations were matched to antimuscarinic drug initiations using a greedy matching algorithm with a ratio up to 1:4Citation24. Matches were restricted to patients who initiated mirabegron or antimuscarinic medication in a given calendar year (and who had not yet matched), and matches were restricted to patients of the same sex and age category (18 to <65 years versus 65 years or older). Weighted standardized differences were calculated to assess the covariate balance in the unmatched and matched cohortsCitation25.
All descriptive, primary and secondary analyses were restricted to the PS-matched cohorts and conducted within each study population. Incidence rates with 95% confidence intervals were calculatedCitation26. Cox proportional hazard models were used to estimate hazard ratios (HRs) to compare cancer outcome incidence rates among matched mirabegron and antimuscarinic medication new users. Separate outcome models were created for each sex-specific composite cancer outcome and each of the ten individual sex-specific cancer outcomes. Pre-specified covariates representing cancer specific risk factors not included in the PS models were added to the individual cancer type outcome models for breast (history of BRCA mutation, where available), colon/rectal (history of inflammatory bowel disease), prostate (history of benign prostatic hyperplasia), and uterine (unopposed estrogen use) cancers.
Descriptive analyses
A cohort flowchart was created to describe the number of mirabegron and antimuscarinic medication initiators identified during the accrual period, the number of patients eligible for matching (overall and by calendar year), the number of patients not eligible for matching (overall and by reason for ineligibility), and the number of matched patients. Summary measures (frequencies, proportions, medians, and interquartile ranges) were used to describe the new users of mirabegron and antimuscarinic medications, and included information on baseline characteristics (before and after matching), the observed number of prescriptions for mirabegron and antimuscarinic medications, the total (given or estimated) days’ supply, and number of days from matched initiation until end of follow-up.
Primary analyses
The primary analyses were ever-treated analyses, in which once patients were exposed to mirabegron, the remainder of their person-time exposure was categorized as mirabegron-exposed, even if they switched to an antimuscarinic medication. In contrast, if antimuscarinic medication initiators subsequently initiated mirabegron, the remaining person-time was categorized as mirabegron-exposed.
The incidence of sex-specific composite cancer outcomes among new users of mirabegron and new users of any comparator antimuscarinic medications (as a group) were estimated and compared, overall and separately for categories of time: person-time in the 1 year following the start of treatment, and person-time in the period >1 year following the start of treatment. The same analysis was performed in patients aged 65 years and older. Additional analyses were conducted to examine protopathic bias, by estimating and comparing the incidence of the ten individual sex-specific cancers included in the composite cancer endpoints among new users of mirabegron and new users of antimuscarinic medication in the follow-up time intervals: 0 to <6 months, 6 to <12 months, 12 to <24 months, and ≥24 months.
Secondary analyses
A series of secondary analyses were conducted to estimate and compare the sex-specific composite cancer outcomes for mirabegron and antimuscarinic medication use, stratified by new user status (i.e. naïve versus non-naïve) and by age groups (18 to ≤44 years, 45 to ≤54 years, 55 to ≤64 years, 65 to ≤74 years, ≥75 years), overall and separately for categories of person-time following the start of treatment. Another secondary analysis estimated and compared the effect of cumulative dose within tertiles of mirabegron dose.
Meta-analysis
A meta-analytic approach was used to estimate pooled HR estimates across study populations. Given anticipated heterogeneity in patient characteristics, prescribing patterns, and availability of covariate information across study populations, both random effects and fixed effects models were implemented, with point estimates representing a weighted HR of the results from the individual study populations and with inverse-variance-weightingCitation27. Heterogeneity across data sources was assessed using the I2 test, with I2 >50% used to indicate substantial heterogeneity.
Results
Matched cohort creation
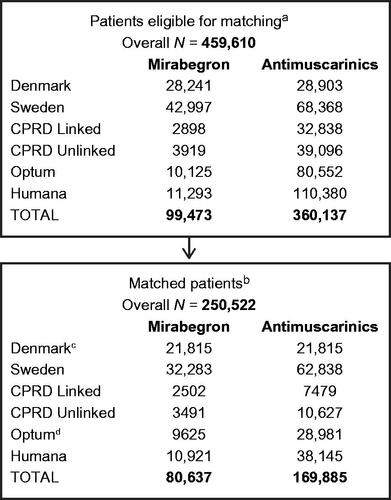
The total number of OAB medication initiators across all data sources who were eligible for PS matching after applying all exclusion criteria was 459,610 (mirabegron [nm] = 99,473 and antimuscarinic medications [na] = 360,137). Only 0.02% of patients initiating an antimuscarinic medication were excluded due to the prescription being for a non-oral formulation. From these, 250,522 patients were matched (nm = 80,637 [32%] and na = 169,885 [68%]) ().
Figure 1. Matched cohort creation for all data sources. aPatients eligible for matching are those with prescription of mirabegron or antimuscarinic medications that met all study inclusion criteria (i.e. not excluded due to the listed exclusion criteria). bMatched patients are those that met all study inclusion/exclusion criteria and were PS-matched at a 1:4 ratio. cOnly 1:1 matching was performed in Denmark. For all other data sources, 1:1 up to 1:4 matching was performed. dAfter case adjudication, 2 patients with adjudicated cancer dates before the index date (mirabegron cohort) were removed, together with their 4 matches, from the final analytic file. Abbreviations. CPRD, Clinical Practice Research Datalink; OAB, overactive bladder.
Descriptive characteristics
PS matching within each data source achieved balance across a range of key measured covariates. Of the 250,522 matched initiators, 169,594 (68%) were 65 years of age or older. Within each data source there was a higher proportion of women than men, ranging from 56% in the Danish database to 74% in the Humana database (). The number of prescriptions, length of follow-up, and reasons for censoring in the mirabegron and matched antimuscarinic medication cohorts varied across data sources (data not presented). The proportion of patients with prior antimuscarinic medication use for the matched mirabegron and antimuscarinic medications cohorts, respectively, were broadly similar in the Danish (13.1%, 12.7%), Swedish (9.5%, 5.2%), Optum (11.1%, 8.0%), and Humana (11.3%, 8.7%) databases; CPRD-linked (45.8%, 34.1%) and CPRD-unlinked (47.2%, 33.6%) databases had higher proportions. The median total days’ supply for mirabegron ranged from 90 days (Humana data) to 300 days (Danish data), while for antimuscarinic medications the range was from 88 days (CPRD-linked data) to 210 days (Danish data). The longest median follow-up time was in the Danish data for the mirabegron cohort (1019 days); the shortest was in the ORD for the antimuscarinic medications cohort (334 days).
Table 1. Baseline characteristics of mirabegron and antimuscarinic initiators in propensity score matched cohorts, all study populations.
Results of the primary analyses
There was no substantial heterogeneity observed across data sources for each of the primary analyses. Results for the fixed effects and random effects models were generally similar, therefore only results from the fixed effects models are provided below and those for the random effects model are provided in Supplementary Table S1. shows meta-analyses results for the sex-specific composite cancer endpoints. Over 5000 incident cancer cases were observed across the five data sources: 2750 in women and 3085 in men. Cancer incidence rates (per 1000 person-years [PY]) for composite cancer outcomes in mirabegron and antimuscarinic medication cohorts, respectively, were 10.09 (95% CI: 9.53, 10.67) and 8.71 (8.28, 9.16) in women, and 21.01 (19.91, 22.15) and 18.99 (18.12, 19.90) in men, for all ages. Women, in general, had similar composite cancer incidence rates in the two follow-up periods since treatment initiation (first year and excluding first year). For men, higher composite cancer incidence rates were observed in the first year of treatment initiation.
Table 2. Meta-analysis results for hazard ratios of sex-specific composite cancer endpoints, propensity-score matched cohorts, all ages, all study populations by time since treatment initiation.
Overall, our results for both men and women when analyzing mirabegron compared with antimuscarinic use and the risk of cancer indicated that all HRs were close to 1, with CIs including 1 (). The overall HR was 1.05 (95% CI: 0.98, 1.14) for women and 1.06 (0.98, 1.14) for men. Overall results by data source were generally similar, with HRs across data sources ranging from 0.84 to 1.34 for women and from 0.83 to 1.16 for men (Supplementary Figure S1).
Among patients 65 years or older, there was also no apparent association between mirabegron use and the risk of cancer, compared with antimuscarinic medication use, in either men or women. HRs overall, in the first year of treatment initiation and excluding the first year of treatment initiation, respectively, for those aged 65 years or older were 1.06 (95% CI: 0.97, 1.16), 1.06 (0.93, 1.19), and 1.05 (0.93, 1.19) for women and 0.99 (0.91, 1.07), 0.95 (0.86, 1.06), and 1.03 (0.91, 1.16) for men.
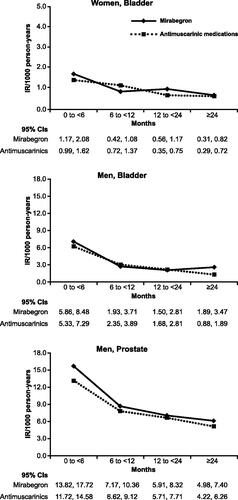
Cancer incidence rates (per 1000 PY) for bladder cancer in both sexes and prostate cancer in men, stratified by intervals of time since cohort entry in order to assess protopathic bias, are summarized in . Cancer incidence rates (per 1000 PY) for each of the other individual cancer types included in the sex-specific composite cancer endpoints are summarized in Supplementary Figures S2 and S3. Overall, for both sexes and for mirabegron and antimuscarinic medications, the highest incidence rates of bladder cancer were observed in the first 6 months compared with later time periods; among men, a decline in prostate cancer incidence rates after the first 6 months was also apparent. For all the other types of cancers, the incidence rates were generally similar across time intervals.
Figure 2. Summary of incidence rates for bladder cancer in women and men and prostate cancer in men, propensity-score matched cohorts, stratified by time since treatment initiation, all data sources. Abbreviation. IR, incidence rate.
The results for the individual cancer outcomes investigating an association between the use of mirabegron, compared with use of antimuscarinic medications, generally showed 95% CIs for the HRs including 1. For women, an association was observed with overall risk of melanoma (HR = 1.47; 95% CI: 1.05, 2.07). The association was stronger in the time period immediately after treatment initiation (first year HR = 2.34; 95% CI: 1.29, 4.24, and 0 to <6 months HR = 3.33; 95% CI: 1.36, 8.15). For men, there was no observed association with risk of melanoma.
Mirabegron was not associated with the risk of colon/rectal cancer among women overall. An association was observed for analyses excluding the first year of follow-up (HR = 1.40; 95% CI: 1.06, 1.85) and 12 to <24 months after treatment initiation (HR = 1.91; 95% CI: 1.31, 2.80) (Supplementary Table S2). For men, there was no observed association with risk of colon/rectal cancer.
In men, there was no evidence of an association between mirabegron and prostate cancer. For bladder cancer, there was no evidence of an association with mirabegron in women; in men, an HR of 1.91 (95% CI: 1.13, 3.21) was observed for the period ≥24 months since treatment initiation, but not for the other comparisons.
Secondary results
Results from secondary analyses overall were similar to those observed in the primary analyses. The HR for naïve new users was 1.03 (95% CI: 0.94, 1.12) for women and 1.06 (0.98, 1.15) for men. For non-naïve new users, the HR was 1.15 (95% CI: 0.94, 1.41) for women and 1.03 (0.83, 1.29) for men. HR estimates stratified by time since treatment initiation were similar. For the analyses stratified by age groups, the incidence rates for women and men increased in a consistent manner from youngest to oldest age groups. HRs remained around 1 for patients ≥55 years; for younger age groups (<55 years), most HRs tended to be numerically higher than those in the older age groups across all the time intervals, but CIs tended to be wide and all included 1. An increase in the risk of cancer was observed among the sub-group of men 45 to ≤54 years who used mirabegron compared with antimuscarinic medications (HR = 1.79; 95% CI: 1.07, 3.00). This association was present in the first year of treatment initiation (HR = 2.41; 95% CI: 1.20, 4.81), but was no longer present after the first year of treatment (HR = 1.26; 95% CI: 0.58, 2.74).
Cumulative dose analysis results
The most commonly prescribed antimuscarinic medications were solifenacin in the Danish, CPRD-linked and CPRD-unlinked databases, tolterodine in the Swedish National Registers, and oxybutynin in the US data sources (ORD and Humana). The median cumulative dose for mirabegron was 12,000 mg in the Danish data; 6000 mg in the Swedish data; 4700 mg in CPRD-linked data; 6650 mg in CPRD-unlinked data; and 3000 mg in the Optum and Humana databases.
Results for sex-specific composite cancer endpoints across tertiles of mirabegron cumulative dose are given in ; medium and high doses were compared with the low dose. The HRs for women were 1.11 (95% CI: 0.97, 1.27) and 1.13 (0.97, 1.32) for medium and high doses, respectively. Among men, HRs were 0.86 (95% CI: 0.76, 0.98) and 0.74 (0.63, 0.86) for those exposed to medium and high cumulative doses of mirabegron, respectively.
Table 3. Hazard ratios of sex-specific composite cancer endpoints, a comparison within tertiles of mirabegron cumulative dose.
Discussion
The goal of this study was to generate robust evidence to assess the risk of cancer among patients treated with mirabegron for OAB, compared with the risk for patients treated with antimuscarinic medications. Employing databases covering a large and diverse group of patient populations, the primary analyses showed no indication of a meaningful association between mirabegron use and risk of cancer outcomes, compared with exposure to antimuscarinic medications, in either men or women. It should be noted that previous studies of antimuscarinic agents used to treat patients with OAB have also observed no increased cancer risk associated with their useCitation28–30.
Our results, indicating no increase in cancer risk with mirabegron, were similar across all patient groups analyzed in this study, including the overall population and patients 65 years of age or older. Furthermore, there was no evidence of any meaningful associations between mirabegron use and risk of cancer outcomes within the first year of treatment initiation, compared with exposure to antimuscarinic medications. This is reassuring, as the PMR to evaluate cancer risks associated with use of mirabegron that led to the current study was based on numerical imbalances observed following short-term use of mirabegron during phase 2/3 clinical trials.
This study found an association between mirabegron and melanoma in women but not in men. Given this inconsistent finding between women and men, and no known plausible mechanism of action to support a carcinogenic or tumor promoting effect of mirabegron on melanoma, and given the large number of contrasts made for the composite and individual cancer outcomes, this finding is considered likely to be due to chance.
The higher incidence rates for bladder and prostate cancers for both cohorts observed in the first 6 months after treatment initiation appear to be the result of protopathic bias, i.e. due to symptoms of bladder and prostate cancer being misinterpreted as overactive bladder symptoms. An apparent increased incidence shortly after treatment initiation followed by normalization of the incidence has also been observed in cancer-risk studies of antimuscarinic agents and has likewise been attributed to protopathic biasCitation29,Citation30. The observed decrease following the initial increase in incidence rates for prostate and bladder cancer across the treatment initiation periods were generally non-differential between mirabegron and antimuscarinic medication use, with all HRs under 2.0 and most of the CIs for the HRs including unity. The exception was bladder cancer in men ≥24 months after treatment initiation (HR = 1.91, 95% CI: 1.13, 3.21).
In some sub-analyses, we observed an increase in risk of colon/rectal cancer among women exposed to mirabegron compared with those exposed to antimuscarinic medications. Of note, colon cancer typically shows symptoms for some time before the actual diagnosis takes place.Citation31 Thus, any associations observed between mirabegron and colon cancer in this study should be cautiously interpreted given the generally long cancer development process and the relatively short overall duration of follow-up in the data sources. The exception to this rule would be cancers related to immunosuppression (e.g. lymphoma); however, associations were not observed for such cancers.
The greatest cumulative dose for mirabegron was observed in Denmark, where the longest median follow-up time was also observed. A lower risk of cancer was observed among men for medium and high cumulative doses of mirabegron relative to low cumulative dose. The size of this reduced risk, however, diminished in the analysis that excluded bladder and prostate cancers from the composite cancer endpoints [results not shown], which is consistent with the presence of protopathic bias. For the secondary analysis that stratified the incidence of sex-specific composite cancer endpoints by age groups, the observed increase in incidence rates with increasing age is consistent with the epidemiology of cancer in the general population. Among men aged 18 to ≤44 years, the HR was approximately 2, even though the respective cancer incidence rates associated with mirabegron and antimuscarinic medications were almost the same. This could probably reflect non-proportional hazards that led to divergence between the risk ratio and hazard ratio estimates.
This study had several major strengths. One of the strengths is the large, multinational source population base used to investigate the risk of neoplasms in association with drugs for OAB in a variety of real-world clinical practice settings, providing a broad array of patient characteristics, drug utilization and real-world medical practice patterns. In this way, the study may be highly generalizable since it reflects both extensive and wide-ranging use. The measure of exposure (to mirabegron or antimuscarinics) was derived from automated records of pharmacy dispensing, or prescriptions written by general practitioners, so that it was not dependent on patient or provider recall. Furthermore, since some of the data sources recorded prescriptions dispensed rather than prescriptions issued, this also meant that in these cases, primary non-compliance was not present in most populations. Finally, the occurrence of the study outcome (cancer) was confirmed via direct linkage to cancer registers or validated through diagnostic claims followed up by medical chart adjudication or physician questionnaires.
The study had several limitations, including a relatively short follow-up time for the study of cancer outcomes, and the fact that while the study assessed the risk of ten common cancers, risk for rarer cancers was not evaluated. In addition, patient lifestyle factors that might have influenced cancer risk were not available from all of the data sources. It should also be noted that there were differences in duration of history and follow-up between data sources. For example, since the whole population is included in the Danish and Swedish registers, the only way to leave the databases is to die or emigrate from the country. In contrast, in US claims databases, duration of history and follow-up may be limited due to individuals changing health insurance plans relatively regularly. Similarly, for CPRD, follow-up is truncated when patients move and enroll with a practice that does not contribute data to the CPRD. Another limitation is related to how exposure was identified in the data sources. The presence of a record of a prescription dispensed or a prescription issued does not necessarily indicate that the medication was consumed or that it was taken as prescribed. However, this is not expected to be differential by mirabegron or antimuscarinic use, and it is also unlikely that patients would repeatedly redeem prescriptions for drugs they did not use since our exposure metric was ever-treated with the drugs and the focus was on long-term use.
Conclusions
Results from the present study suggest no association between mirabegron use and risk of cancer, compared with antimuscarinic medications, in either men or women. Given the robust approach undertaken, and the diverse nature of the study population, these findings are likely to be generalizable to the broader population of mirabegron users in real-world situations with similar use patterns and healthcare access.
Mirabegron PMR-PASS study group
Optum: Cheryl Enger, Kelesitse Phiri (former Optum employee), Veena Hoffman (former Optum employee), John Seeger
University of Southern Denmark: Jesper Hallas, Morten Olesen, Nina Sahlertz Kristiansen
Centre for Pharmacoepidemiology: Shahram Bahmanyar, Marie Linder, Ingvild Odsbu (former employee), Helle Kieler
RTI Health Solutions, Barcelona, Spain: Alejandro Arana, Andrea Margulis, Susana Perez-Gutthann; RTI Health Solutions, Research Triangle Park, North Carolina, USA: Lisa McQuay, Ryan Ziemiecki
Humana Healthcare Research: Su Bunniran, Libby Horter (former Humana employee), Brandon Suehs, Claudia Uribe, Yihua Xu
Astellas Pharma: Kwame Appenteng, Stefan de Vogel, Noah Jamie Robinson, Songlin Xue, Josie Wolfram, Achim Steup, Jena Giese-Pagac, Raymond van Aarle, Neha Sheth, David Burns, Natalie Boone, Milbhor D’Silva (former Astellas employee), Billy Franks (former Astellas employee), Willem Jan Atsma (former Astellas employee), Tim Auton (posthumously)
Scientific Advisory Board (SAB): Edeltraut Garbe, Anders Ekbom, Todd Lee, Noel Weiss, John Rumsfeld
Transparency
Declaration of funding
The Mirabegron PMR-PASS study was supported by Astellas Pharma Global Development.
Declaration of financial/other relationships
M.L., I.O. and S.B. were employees at the time of the study of the Centre for Pharmacoepidemiology, Karolinska Institutet, which receives grants from several entities (pharmaceutical companies, regulatory authorities, and contract research organizations), including Astellas and Pfizer, for performance of drug safety and drug utilization studies. K.P and V.H. were employees of Optum; C.E. and J.S. are employees of Optum. K.P., C.E., V.H., J.S. hold stock in UnitedHealth Group, Optum’s parent company; UnitedHealthcare, a UnitedHealth subsidiary, is a major purchaser of pharmaceutical products. The work was funded with a research contract between Optum and Astellas. J.H., M.O. and N.S.K. are employees at Clinical Pharmacology and Pharmacy at the University of Southern Denmark. The university received grants from Astellas for the conduct of this study. No grants were paid directly to any of the researchers. A.A., A.M. and S.P-G. are employees of RTI Health Solutions. RTI Health Solutions is a unit of RTI International, an independent, nonprofit organization that conducts work for government, public, and private organizations, including pharmaceutical companies. The authors participated in this work in the course of employment as work for hire, pursuant to a contract to conduct an independent research study for a client (Astellas). Authors received no compensation other than annual salary from employer. B.S. and L.H. are employees of Humana Healthcare Research, which received funding from Astellas in connection with the performance of this study. S. de V. and K.A. are employees of Astellas. A reviewer on this manuscript has disclosed that they have received payment from Astellas Pharma for consultancy and as a speaker in the past; they have also participated in research on mirabegron. All other peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Author contributions
Conception and design: All authors
Analysis and interpretation of the data: All authors
Drafting of the paper or revising it critically for intellectual content: All authors
Final approval of the version to be published: All authors
All authors agree to be accountable for all aspects of the work.
Supplemental Material
Download MS Word (57.4 KB)Supplemental Material Figure S3
Download PDF (603.8 KB)Supplemental Material Figure S2
Download PDF (604.9 KB)Supplemental Material Figure S1
Download PDF (253.4 KB)Acknowledgements
The authors would like to thank all the Mirabegron PMR-PASS study investigators. Additional thanks are due to Nicole Brooks, Sara Yuewen Gao, Laura Karslake, Nan Liu, Katherine Reed, Bruce Turnbull and Jing Yang (Optum) and Kathleen Mortimer (former Optum employee); Ryan Ziemiecki and Lisa McQuay (statistician-analysts from RTI-Health Solutions) and Christine Bui (epidemiologist from RTI-Health Solutions). This study was funded by Astellas Pharma Global Development. Medical writing support was provided by Sue Cooper and Catherine Elliott of Envision Pharma Group and funded by Astellas Pharma Global Development.
This study is based in part on data from the Clinical Practice Research Datalink obtained under licence from the UK Medicines and Healthcare products Regulatory Agency. The data is provided by patients and collected by the NHS as part of their care and support. The interpretation and conclusions contained in this study are those of the author/s alone. Copyright © (2018), re-used with the permission of The Health & Social Care Information Centre. All rights reserved.
Data availability statement
This observational study is based on individual patient data. Therefore, we are not able to make this data fully available to the public.
References
- Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology. 2003;61(1):37–49.
- Haylen BT, de Ridder D, Freeman RM, International Continence Society, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29(1):4–20.
- Rahnama’i S. Overactive bladder. Current Definition. 2018. [cited 2021 Jan 14]. Available from: https://www.ics.org/committees/standardisation/terminologydiscussions/overactivebladder.
- Drake MJ. Do we need a new definition of the overactive bladder syndrome? ICI-RS 2013. Neurourol Urodyn. 2014;33(5):622–624.
- Willis-Gray MG, Dieter AA, Geller EJ. Evaluation and management of overactive bladder: strategies for optimizing care. Res Rep Urol. 2016;8:113–122.
- Baessler K, Christmann-Schmid C, Maher C, et al. Surgery for women with pelvic organ prolapse with or without stress urinary incontinence. Cochrane Database Syst Rev. 2018;8:CD013108.
- Yamada S, Ito Y, Nishijima S, et al. Basic and clinical aspects of antimuscarinic agents used to treat overactive bladder. Pharmacol Ther. 2018;189:130–148.
- Astellas Pharma US I. MYRBETRIQ prescribing information. 2018. [cited 2021 Jan 14]. Available from: https://www.us.astellas.com/docs/Myrbetriq_WPI.pdf.
- Chapple CR, Kaplan SA, Mitcheson D, et al. Randomized double-blind, active-controlled phase 3 study to assess 12-month safety and efficacy of mirabegron, a β(3)-adrenoceptor agonist, in overactive bladder. Eur Urol. 2013;63(2):296–305.
- Nitti VW, Auerbach S, Martin N, et al. Results of a randomized phase III trial of mirabegron in patients with overactive bladder. J Urol. 2013;189(4):1388–1395.
- Chapple CR, Dvorak V, Radziszewski P, et al. A phase II dose-ranging study of mirabegron in patients with overactive bladder. Int Urogynecol J. 2013;24(9):1447–1458.
- Yamaguchi O, Marui E, Igawa Y, et al. Efficacy and safety of the selective beta3-adrenoceptor agonist mirabegron in Japanese patients with overactive bladder: a randomized, double-blind, placebo-controlled, dose-finding study. Lower Urinary Tract Symptoms. 2015;7(2):84–92.
- Yamaguchi O, Marui E, Kakizaki H, et al. Phase III, randomised, double-blind, placebo-controlled study of the beta3-adrenoceptor agonist mirabegron, 50 mg once daily, in Japanese patients with overactive bladder. BJU Int. 2014;113(6):951–960.
- Herschorn S, Barkin J, Castro-Diaz D, et al. A phase III, randomized, double-blind, parallel-group, placebo-controlled, multicentre study to assess the efficacy and safety of the β3 adrenoceptor agonist, mirabegron, in patients with symptoms of overactive bladder. Urology. 2013;82(2):313–320.
- Khullar V, Amarenco G, Angulo JC, et al. Efficacy and tolerability of mirabegron, a beta(3)-adrenoceptor agonist, in patients with overactive bladder: results from a randomised European-Australian phase 3 trial. Eur Urol. 2013;63(2):283–295.
- Public Policy Committee. International Society of Pharmacoepidemiology. Guidelines for good pharmacoepidemiology practice (GPP). Pharmacoepidemiol Drug Saf. 2016;25(1):2–10.
- The European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP). Guide on Methodological Standards in Pharmacoepidemiology (Revision 5). 2020. [cited 2021 Jan 14]. Available from: http://www.encepp.eu/standards_and_guidances.
- Centers for Disease Control and Prevention. International Classification of Diseases, Ninth Revision (ICD-9). 2020. [cited 2021 Jan 14]. Available from: https://www.cdc.gov/nchs/icd/icd9.htm.
- Centers for Disease Control and Prevention. International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM). [cited 2021 Jan 14]. Available from: https://www.cdc.gov/nchs/icd/icd10cm.htm.
- Centers for Disease Control and Prevention. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). 2020. [cited 2021 Jan 14]. Available from: https://www.cdc.gov/nchs/icd/icd9cm.htm.
- World Health Organization. International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3). 2020. [cited 2021 Jan 14]. Available from: https://www.who.int/classifications/icd/adaptations/oncology/en/.
- Surveillance, Epidemiology and End Results. SEER Cancer Statistics Review 1975–2009 (vintage 2009 populations). Table 1.4. Age-adjusted SEER incidence and US death rates and 5-year relative survival (percent) by primary cancer site, sex, and time period. [cited 2021 Jan 14]. Available from: https://seer.cancer.gov/archive/csr/1975_2009_pops09/browse_csr.php.
- Eurostat. Statistics Explained. Cancer statistics – specific cancers. 2019. [cited 2021 Jan 14]. Available from: https://ec.europa.eu/eurostat/statistics-explained/pdfscache/39738.pdf.
- Parsons LS. Reducing bias in a propensity score matched-pair sample using greedy matching techniques (paper 214–26). Paper presented at: SUGI 26 Proceedings (Proceedings of the 26th annual SAS users group international conference), Long Beach, CA, April 22–25, 2001. 2001. [cited 2021 Jan 14]. Available from: https://support.sas.com/resources/papers/proceedings/proceedings/sugi26/p214-26.pdf
- Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–3107.
- Rothman KJ. Modern epidemiology. 3rd ed. Philadelphia (PA): Wolters Kluwer Health; 2008.
- Comprehensive Meta-Analysis (CMA) Software. [cited 2021 Jan 14]. Available from: https://www.meta-analysis.com/.
- Lofling L, Sundstrom A, Kieler H, et al. Exposure to antimuscarinic medications for treatment of overactive bladder and risk of lung cancer and colon cancer. CLEP. 2019;11:133–143.
- Hallas J, Margulis AV, Pottegard A, et al. Incidence of common cancers in users of antimuscarinic medications for overactive bladder: A Danish nationwide cohort study. Basic Clin Pharmacol Toxicol. 2018;122(6):612–619.
- Kaye JA, Margulis AV, Fortuny J, et al. Cancer incidence after initiation of antimuscarinic medications for overactive bladder in the United Kingdom: evidence for protopathic bias. Pharmacotherapy. 2017;37(6):673–683.
- Gorin SS. Multilevel approaches to reducing diagnostic and treatment delay in colorectal cancer. Ann Fam Med. 2019;17(5):386–389.

