Abstract
Background
The clinicopathological factors indicating risk of recurrence are used to guide the choice of perioperative therapy in patients with breast cancer. Although several risk factors for recurrence have been reported in patients with hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (HER2−) early breast cancer in Japan, there has been no systematic review quantifying potential risk factors.
Methods
We performed a systematic literature review and meta-analysis using the MEDLINE, Embase, Cochrane CENTRAL, and Japan Medical Abstract Society databases to identify risk factors for recurrence in HR+/HER2− early breast cancer in Japan. The primary outcome was relapse-free or disease-free survival (RFS/DFS), and the secondary outcomes were overall survival and breast cancer-specific survival (BCSS).
Results
Searches identified 42 eligible publications. Meta-analyses identified lymph node metastasis (hazard ratio: 2.76 [95% confidence interval: 1.97–3.88]), large tumor size (1.67 [1.24–2.23]), high histological grade (1.50 [1.04–2.16]), and high nuclear grade (2.02 [1.61–2.54]) as risk factors for RFS/DFS. Lymph node metastasis (2.43 [1.28–4.63]), large tumor size (1.80 [1.24–2.62]), and high histological grade (2.02 [1.44–2.84]) were also risk factors for overall survival, and high progesterone status was a possible favorable prognostic factor for BCSS (0.20 [0.10–0.42]).
Conclusions
Identified risk factors were consistent with the previous reports, and this study provides quantitative summary of risk factors for HR+/HER2– early breast cancer recurrence in Japan. (PROSPERO Registration ID, CRD42022338391.)
Introduction
Breast cancer is the most common cancer among women in the US and JapanCitation1,Citation2. Approximately 92 000 new cases of breast cancer were reported in Japan in 2020Citation2, and the average annual incidence of breast cancer is estimated to increase until 2025Citation3. Breast cancer mortality has also been increasing since the 1970sCitation4.
Nearly 90% of patients have either localized or regional breast cancer at diagnosis, and are commonly treated by surgery in combination with other modalities such as radiation, chemotherapy, hormone therapy, or targeted immunotherapy with curative intentCitation5,Citation6. However, breast cancer is a heterogeneous disease with several molecular/clinical subtypes based on factors such as hormone receptor status and human epidermal growth factor receptor 2 (HER2) protein expression, and the optimal treatment is guided by the expression of biomarkers including estrogen receptor, progesterone receptor (PgR), and HER2Citation7,Citation8. Hormone receptor-positive (HR+), HER2-negative (HER2−) breast cancer is the most prevalent subtype, and is recommended the treatment included hormonal therapies. However, around 30% of HR+/HER2− patients with certain features in terms of lymph node metastasis, tumor size, tumor grade, or other proliferation markers (e.g. Ki-67) are at higher risk of relapsing with incurable metastatic diseaseCitation9–13.
Asian patients with breast cancer exhibit distinct clinicopathological characteristics compared to non-Asian patientsCitation14,Citation15. In the monarchE study that examined the effects of adding abemaciclib to adjuvant endocrine therapy in patients with high-risk HR+/HER2− early breast cancer (EBC), patients from Asia were generally younger, more frequently premenopausal and had a higher incidence of ≥4 positive nodes than those from non-Asian countriesCitation16. As noted above, several factors are known to be associated with the risk of cancer recurrence. However, it is unclear whether the risk factors for recurrence based on Western data contribute to the risk of recurrence to a similar extent in Asians.
In this study, we systematically summarized published evidence regarding the risk factors for breast cancer recurrence and conducted meta-analyses to evaluate their impact on recurrence in patients with HR+/HER2− EBC in Japan.
Methods
This systematic literature review was registered in PROSPERO (International Prospective Register of Systematic Reviews; Registration ID: CRD42022338391; website: http://www.crd.york.ac.uk/PROSPERO/) and conducted based on the preferred reporting items for systematic reviews and meta-analyses guidelines.Citation17
Search strategy
We searched the MEDLINE, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), and the Japan Medical Abstracts Society (JAMAS) databases for relevant publications between January 2010 and June 2022. The search was restricted to studies published in English or Japanese and studies in humans. The detailed search strategies are presented in Supplementary Table 1.
Eligibility criteria
Publications that met the following criteria were included in this systematic review and meta-analyses: studies including early-stage (stage I, II, and III [operable only]) and HR+/HER2− breast cancer patients in Japan; no limitations on interventions or comparators; outcome factors associated with the risk of cancer recurrence or other relevant outcomes included relapse-free survival (RFS) or disease-free survival (DFS), overall survival (OS), and breast cancer-specific survival (BCSS), with RFS or DFS (RFS/DFS) as the outcome of primary interest; observational studies and interventional clinical trials with sample sizes ≥25 patients; and English/Japanese reports published between 1 January 2010 and 22 June 2022. Studies were excluded if <50% of the patient population was either HR + or HER2−. Letters and commentaries were also excluded.
Study selection
Three authors (N. M., T. I., and K. N.) conducted a comprehensive literature search, eliminated duplicate publications, and independently reviewed the titles and abstracts of the studies for possible relevance. The same authors subsequently assessed the full texts of the articles for inclusion. Disagreements among the authors were resolved by discussion among the same three authors.
Risk of bias assessment
The risk of bias (RoB) was assessed using the quality in prognosis studies toolCitation18, which evaluates the validity and bias in studies of prognostic factors using six domains: study participation, study attrition, prognostic factor measurement, outcome measurement, study confounding, and statistical analysis and reporting. The assessment of RoB in each domain was based on multiple low/moderate/high questions. The overall RoB for each article was concluded based on the results of the six domains as follows: low overall RoB, four to six domains classified as low RoB and the rest as moderate RoB; moderate overall RoB, one domain classified as high RoB and the rest as moderate or low RoB, two domains classified as high RoB and the rest as low RoB, or three domains classified as moderate RoB and low RoB; and high overall RoB, all other cases. Two authors (N. M. and K. N.) conducted the RoB evaluation, and any disagreements between these two authors were resolved by discussion among N. M., T. I., and K. N.
Statistical analysis
Meta-analyses were conducted when more than three reports evaluated the relevant risk factor for each endpoint. RFS and DFS were unified as an endpoint RFS/DFS, since RFS and DFS are similar endpoints and are sometimes used interchangeably. Hazard ratios (HRs) were used as effect size measures. Multivariable-adjusted HRs by covariates for risk factors were used for primary analyses, and univariable HRs for risk factors were used for sensitivity analyses. HRs were combined using a random-effects model with the Sidik–Jonkman estimatorCitation19 and Hartung–Knapp’s methodCitation20. Visual inspection of the forest plots was performed, and heterogeneity parameters (I2 and τ2) and their 95% confidence intervals (CIs) were evaluated to investigate the possibility of statistical heterogeneity. An I2 value ≥75% indicated considerable heterogeneity. There are no clear criteria for τ2, and the decision of considerable heterogeneity was therefore based on the estimated average effect. If heterogeneity existed, subgroup analysis of the study characteristics was performed, and the factors used for subgroup analysis were determined by checking forest plots. Influence analyses were performed using Viechtbauer and Cheung’s leave-one-out methodCitation21. If only one or very few studies were responsible for the heterogeneity, a sensitivity analysis was performed, excluding the study or group of studies. Funnel plots and Egger’s test were used to assess publication bias. If standard errors were not reported, they were calculated from the standard deviation, P-values, or CIs and if CIs were not reported, they were calculated from standard errors or P-values. If the data to be synthesized were missing, the study was excluded. For the overall analyses, sensitivity analyses were conducted including only the studies with 100% HR+/HER2− patients. Statistical analyses were carried out using R version 4.2.0 (R Foundation for Statistical Computing; Vienna, Austria).
Results
Study selection
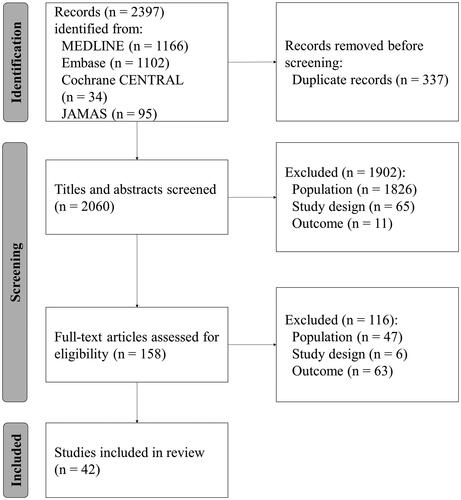
We conducted a systematic literature search of the MEDLINE, Embase, Cochrane CENTRAL, and JAMAS databases up to June 2022. A total of 2397 reports were identified (MEDLINE: n = 1166, Embase: n = 1102, Cochrane CENTRAL: n = 34, JAMAS: n = 95). After discarding duplicates, the titles and abstracts of 2060 reports were screened, of which 158 were selected for full-text review. Forty-two reports were finally eligible for inclusion (). No reports were added by manual searching.
Study characteristics
The characteristics of the 42 reports are presented in . Three reports were randomized controlled trials and 39 were observational studies, including 33 retrospective and six prospective studies. The evaluated outcomes were RFS/DFS (32 reports), OS (9 reports), and BCSS (11 reports). Twenty-five reports included only HR+/HER2− patients. The potential risk factors evaluated in each study are also summarized in . The RoB was high in 19 reports, moderate in 21, and low in two (, Supplementary Table 2).
Table 1. Characteristics of the 42 articles identified in the systematic literature review.
Meta-analyses
Risk factors for RFS/DFS
Nine factors were eligible for meta-analysis in relation to RFS/DFS (, ): lymph node metastasis, tumor size, Ki-67, adjuvant chemotherapy, PgR status, histological grade, nuclear grade, menopausal status, and age were reported in relation to RFS/DFS in more than three reports using either multivariable or univariable results. Lymph node metastasis, tumor size, histological grade, and nuclear grade were significant risk factors in the multivariable analyses (HR: 2.76, 95% CI: [1.97–3.88], 1.67 [1.24–2.23], 1.50 [1.04–2.16], 2.02 [1.61–2.54], respectively), and the results of the univariable analyses were similar. Ki-67 tended to be a high-risk factor and was a significant risk factor in univariable analysis (2.65 [1.76–4.00]), but not in multivariable analysis (1.50 [0.94–2.41]). Adjuvant chemotherapy was not identified as a risk factor in either multivariable or univariable analyses (0.81 [0.47–1.38], 1.19 [0.54–2.61], respectively). High PgR status was a significant favorable prognostic factor in univariable analysis (0.51 [0.27–0.96]) and tended to be associated with an improved prognosis, but without statistical significance, in multivariable analysis (0.60 [0.29–1.22]). Menopausal status and age were not risk factors in univariable analyses and were not examined in multivariable analyses because there were only three and two reports, respectively.
Figure 2. Forest plots for relationships between various factors and RFS/DFS in multivariable analyses. HRs represented by gray boxes; size of boxes correlated with weight; length of lines represents 95% CIs; diamonds indicate pooled HRs. (A) Lymph node metastasis; yes vs no (reference). (B) Tumor size; large vs small (reference). (C) Ki-67; high vs low (reference). (D) Adjuvant chemotherapy; yes vs no (reference). (E) PgR status; high vs low (reference). (F) Histological grade; high vs low (reference). (G) Nuclear grade; high vs low (reference). PgR: progesterone receptor; RFS/DFS; relapse-free survival or disease-free survival; HR: hazard ratio; CI: confidence interval; HK: Hartung-Knapp.
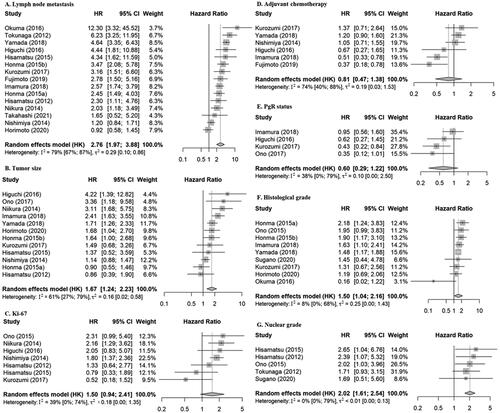
Table 2. Results of meta-analysis by outcomes and factors.
In the primary analyses using multivariable results, only lymph node metastasis showed high heterogeneity (I2 = 79%) () and therefore we underwent subgroup analysis to explore the cause of the heterogeneity (Supplementary Figure 1). We could only perform a subgroup analysis for perioperative chemotherapy (Yes/No), because other factors were not consistently reported. The Yes group included 13 reports, with a pooled HR of 2.83 [2.08–3.85], and the No group only included two reports, resulting in a very wide CI for the pooled HR, which precluded further evaluation. In the influence analyses for the primary analyses using multivariable results, all leave-one-out results were included in the original pooled effect size of 95% CIs, and no reports caused heterogeneity, and sensitivity analyses based on the influence analysis were therefore not performed (Supplementary Figure 2). The meta-analysis results for publishing bias showed no significant publication biases (Supplementary Figure 3). The sensitivity analyses using only studies with 100% HR+/HER2− patients were consistent with the primary analyses (Supplementary Table 3).
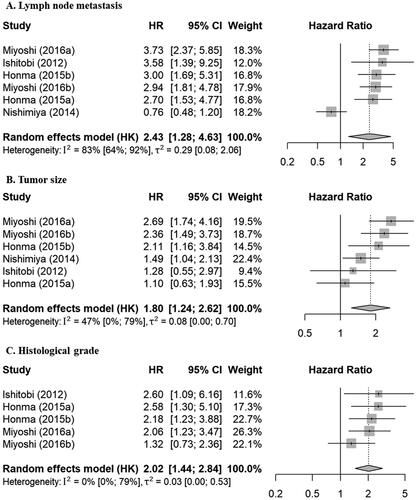
Risk factors for OS
Three factors were eligible for meta-analyses in relation to OS (, ): lymph node metastasis, tumor size, and histological grade were reported in relation to OS in more than three reports with either multivariable or univariable results. Lymph node metastasis and tumor size were significant risk factors in both multivariable (2.43 [1.28–4.63], 1.80 [1.24–2.62], respectively) and univariable analyses (3.89 [2.76–5.47], 2.48 [1.34–4.57], respectively). Histological grade was a significant risk factor in multivariable analysis (2.02 [1.44–2.84]), but only three reports included univariable results.
Figure 3. Forest plots for relationships between various factors and OS in multivariable analyses. HRs represented by gray boxes; size of boxes correlated with weight; length of lines represents 95% CIs; diamonds indicate pooled HRs. (A) Lymph node metastasis; yes vs no (reference). (B) Tumor size; large vs small (reference). (C) Histological grade; high vs low (reference). OS: overall survival; HR: hazard ratio; CI: confidence interval; HK: Hartung-Knapp.
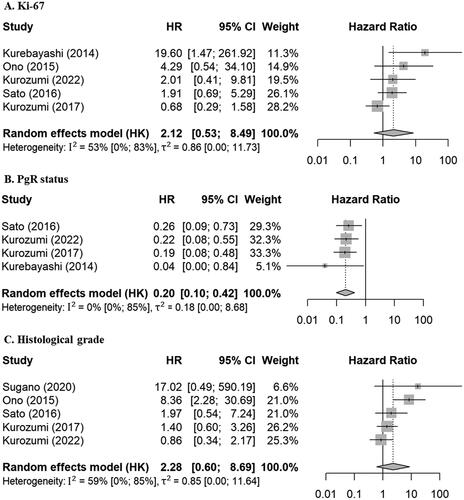
Risk factors for BCSS
Four factors were eligible for meta-analyses in relation to BCSS (, ): tumor size, Ki-67, PgR status, and histological grade were reported in relation to BCSS in more than three reports with either multivariable or univariable results. Ki-67 and histological grade were strong risk factors in univariable analyses (3.76 [1.79–7.93], 5.63 [2.24–14.13], respectively) and tended to be high-risk factors in multivariable analyses, but the results were not significant (2.12 [0.53–8.49], 2.28 [0.60–8.69], respectively). High PgR status was a significant favorable prognostic factor in both multivariable and univariable analyses (0.20 [0.10–0.42], 0.14 [0.03–0.73], respectively). Tumor size was a significant risk factor in univariable analysis (2.39 [1.17–4.87]), but only three reports included multivariable results.
Figure 4. Forest plots for relationships between various factors and BCSS in multivariable analyses. HRs represented by gray boxes; sizes of boxes correlated with weight; lengths of lines represent 95% CIs; diamonds indicate pooled HRs. (A) Ki-67; high vs low (reference). (B) PgR status; high vs low (reference). (C) Histological grade; high vs low (reference). PgR: progesterone receptor; BCSS: breast cancer-specific survival; HR: hazard ratio; CI: confidence interval; HK: Hartung-Knapp.
Discussion
We conducted a systematic literature review and meta-analyses of recurrence risk factors in patients with HR+/HER2− EBC in Japan. The identified risk factors were consistent with those used in the molecular classification of HR+/HER2− EBC into luminal A/B subtypes, and with the risk factors indicated in the existing breast cancer guidelines.Citation6 The results of this study provide a quantitative summary, which can be used to assess the relative importance of each risk factor in patients with breast cancer in Japan. Lymph node metastasis, tumor size, and histological grade were consistently identified as risk factors for RFS/DFS and OS. Regarding BCSS, histological grade was a significant risk factor in univariable but not in multivariable analysis, but showed a consistent trend toward high risk. Lymph node metastasis is a well-known risk factorCitation6, and accordingly had the highest HR among these three factors in the present study.
Ki-67 was a significant risk factor for RFS/DFS and BCSS in univariable analyses but not in multivariable analyses. This apparent discrepancy may be attributable to different methods of evaluation among studies, given that a consensus Ki-67 threshold for predicting risk of recurrence is not well established and testing and assessment methods are not standardizedCitation64; indeed, most identified in this review had thresholds that varied from 10%–30%. Another potential explanation is that Ki-67 is a marker of cell proliferation, which is a fundamental biological process, and it may therefore be confounded by other relevant risk factors.
Abemaciclib has recently been approved for postoperative adjuvant therapy in high-risk patients based on the monarchE trialCitation65, with high risk defined as having at least four positive pathologic axillary lymph nodes or up to positive three axillary lymph nodes plus tumor size ≥5 cm, histologic grade 3 disease, or centrally assessed Ki-67 ≥ 20%. This review also shows that it is reasonable to define high risk according to lymph node metastasis, tumor size, and histological grade.
Overall, PgR status was suggested to be a favorable prognostic factor; it was a significant favorable prognostic factor for RFS/DFS in univariable analyses but not in multivariate analyses, and a significant favorable prognostic factor for BCSS in both analyses. The Japanese Breast Cancer Society Clinical Practice Guidelines and the American Society of Clinical Oncology/College of American Pathologists guidelines also recommend PgR testing to evaluate the risk of future breast cancer, and the results were consistent with the global consensus of cliniciansCitation6,Citation66.
Adjuvant chemotherapy was not associated with a low recurrence risk. The use of adjuvant chemotherapy is at physician’s discretionCitation6, and perioperative chemotherapy regimens have evolved with time. Study periods were 2000–2001 in Kurozumi (2017)Citation43, 1992–2010 in Yamada (2018)Citation48, 1995–1999 in Nishimiya (2014)Citation32, 2008–2014 in Higuchi (2016)Citation38, 2004–2014 in Imamura (2018)Citation46, and 2008–2017 in Fujimoto (2019)Citation50, which indicates variability across studies. Due to the variability in perioperative chemotherapy regimens over time, the limited number of studies, and variations in the factors considered in multivariable analyses across different studies, no definitive difference could be obtained. This factor is, in addition, a posteriori in terms of recurrence risk assessment, and the results of each report should be interpreted with caution.
Breast cancer in Asians is characterized by early tumor onsetCitation2, i.e. young age and pre-menopausal status, but menopausal status and age were only reported in limited numbers of studies in this review, and were not identified as significant risk factors for RFS/DFS. Although young-onset breast cancer has been reported to be more aggressive and have a poorer prognosis, the lack of a clear definition of risk classification by ageCitation67 and relatively older age thresholds (50 or 57 years) in the studies included in this review preclude an adequate discussion of these factors.
Domestic guidelines list lymphovascular invasion as a risk factor for breast cancer recurrenceCitation6; however, few reports in this review assessed it as a potential risk factor, and it was therefore not included in the current meta-analyses.
This systematic review and meta-analysis had some limitations. Some of the risk factors may be missed since most of the original studies are retrospective observational studies. For example, variables like the age of onset and BMI are known to differ in Japanese from Western people like Americans, so can affect outcomes differently in Japanese population. However, most of the 42 studies included in this systematic review did not report the age at diagnosis and BMI, thus it was not possible to perform analysis on these factors. The population characteristics differed between studies, the list of assessed risk factors varied among studies, and the adjustments in multivariable analyses also differed. Meta-analyses using the results of univariable analyses were therefore performed as sensitivity analyses, which confirmed and supplemented the primary analyses. In addition, this study included reports in which >50% of the patients were HR+/HER2−, and 17 reports used in the primary analysis included some non-HR+/HER2− patients. However, we checked the robustness of the results by performing a sensitivity analysis using only the 25 reports in which all patients were HR+/HER2−, and these results were consistent with the primary analyses. Although ER and HER2 were also assessed as risk factors in some of the mixed population studies, these were not relevant to the current study and were therefore omitted from the analyses. Early and late recurrence could not be compared because only two reports included this information. Adjuvant endocrine therapy and radiation therapy also could not be evaluated either because the number of reports included was three and one, respectively. The cause of the high heterogeneity in lymph node metastasis was unclear because we could not perform detailed subgroup analyses due to a lack of information. Different thresholds were used for some risk factors, or groupings were made in each report; for example, some reports treated risk factors as positive vs. negative, while others classified them as grades 0–4. In this study however, we classified and analyzed all risk factors as single binary variables, e.g. high vs low. In addition, most studies included in this systematic review were observational studies, and most ROB scores were moderate or high.
Conclusion
In conclusion, the risk factors identified in the systematic review and meta-analyses were consistent with body of knowledge, including the Japanese Breast Cancer Society Clinical Practice Guidelines, but the current results provide novel information quantifying each risk factor in Japanese patients with HR+/HER2− EBC. Notably, the choice of postoperative adjuvant treatment is based on an assessment of recurrence risk. Accurate assessment of recurrence risk followed by decision of treatment strategies based on it are necessary to improve the clinical outcome of the patients. The results of this study will support these aims and help in the development of better treatment strategies.
Transparency
Declaration of funding
This study was funded by Eli Lilly Japan and outsourced from Eli Lilly Japan to the Biostatistics Unit of Keio University Hospital.
Author contributions
Y. T., Z. C., and T. K. contributed to the study conceptualization and design. N. M., T. I., and K. N. performed a literature search and contributed to data acquisition. K. N. performed statistical analyses. All authors contributed to the interpretation of data. N. M. contributed to manuscript drafting. All authors critically reviewed and revised the draft manuscript and approved the final version for submission.
SupplementaryMaterials.docx
Download MS Word (1.2 MB)Acknowledgements
None.
Declaration of financial/other relationships
H. S. has received speaker’s honorarium and medical writing support from Daiichi Sankyo as well as research funding from Eisai. Y. T, Z. C., and T.K. are employees of Eli Lilly Japan, and minor stockholders of Eli Lilly and Company. J. T.’s institution received study grants from Eli Lilly, Eisai, Daiichi Sankyo, Kyowa Kirin, Taiho, MSD, and Nihon Kayaku, Ono, the West Japan Oncology Group and he was paid personal fee for lecture by the Eli Lilly, Eisai, Daiichi Sankyo, Kyowa Kirin, Taiho, MSD, and Nihon Kayaku Japan.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Data availability statement
The datasets used and/or analyzed during this study are available from the corresponding author upon reasonable request.
References
- NCI. General Information About Breast Cancer. 2023. [cited 2023 Feb 9]. Available from: https://www.cancer.gov/types/breast/hp/breast-treatment-pdq#section/_1.
- IARC. Cancer Today. 2020. [cited 2023 Feb 9]. Available from: https://gco.iarc.fr/today/home.
- Toyoda Y, Tabuchi T, Nakayama T, et al. Past trends and future estimation of annual breast cancer incidence in Osaka, Japan. Asian Pac J Cancer Prev. 2016;17:2847–2852.
- Kawamura T, Sobue T. Comparison of breast cancer mortality in five countries: France, Italy, Japan, the UK and the USA from the WHO mortality database (1960–2000). Jpn J Clin Oncol. 2005;35(12):758–759. doi: 10.1093/jjco/hyi201.
- Yoshimura A, Ito H, Nishino Y, et al. Recent improvement in the long-term survival of breast cancer patients by age and stage in Japan. J Epidemiol. 2018;28(10):420–427. doi: 10.2188/jea.JE20170103.
- Japanese Breast Cancer Society. The Japanese breast cancer society clinical practice guidelines for breast cancer 2022 treatment. Tokyo: Kanehara Shuppan; 2022.
- Shimoi T, Nagai SE, Yoshinami T, et al. The Japanese breast cancer society clinical practice guidelines for systemic treatment of breast cancer, 2018 edition. Breast Cancer. 2020;27(3):322–331. doi: 10.1007/s12282-020-01085-0.
- Gradishar WJ, Moran MS, Abraham J, et al. Breast cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20(6):691–722. doi: 10.6004/jnccn.2022.0030.
- Gerber B, Freund M, Reimer T. Recurrent breast cancer: treatment strategies for maintaining and prolonging good quality of life. Dtsch Arztebl Int. 2010;107(6):85–91. doi: 10.3238/arztebl.2010.0085.
- O'Shaughnessy J. Extending survival with chemotherapy in metastatic breast cancer. Oncologist. 2005;10 Suppl 3(S3):20–29. doi: 10.1634/theoncologist.10-90003-20.
- Cheng L, Swartz MD, Zhao H, et al. Hazard of recurrence among women after primary breast cancer treatment—a 10-year follow-up using data from SEER-Medicare. Cancer Epidemiol Biomarkers Prev. 2012;21(5):800–809. doi: 10.1158/1055-9965.EPI-11-1089.
- Richman J, Dowsett M. Beyond 5 years: enduring risk of recurrence in oestrogen receptor-positive breast cancer. Nat Rev Clin Oncol. 2019;16(5):296–311. doi: 10.1038/s41571-018-0145-5.
- Győrffy B, Hatzis C, Sanft T, et al. Multigene prognostic tests in breast cancer: past, present, future. Breast Cancer Res. 2015;17(1):11. doi: 10.1186/s13058-015-0514-2.
- Leong SP, Shen ZZ, Liu TJ, et al. Is breast cancer the same disease in Asian and Western countries? World J Surg. 2010;34(10):2308–2324. doi: 10.1007/s00268-010-0683-1.
- Agarwal G, Pradeep PV, Aggarwal V, et al. Spectrum of breast cancer in Asian women. World J Surg. 2007;31(5):1031–1040. doi: 10.1007/s00268-005-0585-9.
- Jiang Z, Nakayama T, Nakamura S, et al. Baseline characteristics of patients from Asia enrolled in monarchE, evaluating abemaciclib in high risk early breast cancer. Ann Oncol. 2020;31(S6):S1241. doi: 10.1016/j.annonc.2020.10.022.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–e34. doi: 10.1016/j.jclinepi.2009.06.006.
- Hayden JA, van der Windt DA, Cartwright JL, et al. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158(4):280–286. doi: 10.7326/0003-4819-158-4-201302190-00009.
- Sidik K, Jonkman JN. A simple confidence interval for meta-analysis. Stat Med. 2002;21(21):3153–3159. doi: 10.1002/sim.1262.
- Hartung J, Knapp G. A refined method for the meta-analysis of controlled clinical trials with binary outcome. Stat Med. 2001;20(24):3875–3889. doi: 10.1002/sim.1009.
- Viechtbauer W, Cheung MW. Outlier and influence diagnostics for meta-analysis. Res Synth Methods. 2010;1(2):112–125. doi: 10.1002/jrsm.11.
- Shibuta K, Ueo H, Furusawa H, et al. The relevance of intrinsic subtype to clinicopathological features and prognosis in 4,266 Japanese women with breast cancer. Breast Cancer. 2011;18(4):292–298. doi: 10.1007/s12282-010-0209-6.
- Tokiniwa H, Horiguchi J, Takata D, et al. Topoisomerase II alpha expression and the Ki-67 labeling index correlate with prognostic factors in estrogen receptor-positive and human epidermal growth factor type-2-negative breast cancer. Breast Cancer. 2012;19(4):309–314. doi: 10.1007/s12282-011-0291-4.
- Hisamatsu Y, Tokunaga E, Yamashita N, et al. Impact of FOXA1 expression on the prognosis of patients with hormone receptor-positive breast cancer. Ann Surg Oncol. 2012;19(4):1145–1152. doi: 10.1245/s10434-011-2094-4.
- Ishitobi M, Ohsumi S, Inaji H, et al. Ipsilateral breast tumor recurrence (IBTR) in patients with operable breast cancer who undergo breast-conserving treatment after receiving neoadjuvant chemotherapy: risk factors of IBTR and validation of the MD Anderson prognostic index. Cancer. 2012;118(18):4385–4393. doi: 10.1002/cncr.27377.
- Masuda N, Sagara Y, Kinoshita T, et al. Neoadjuvant anastrozole versus tamoxifen in patients receiving goserelin for premenopausal breast cancer (STAGE): a double-blind, randomised phase 3 trial. Lancet Oncol. 2012;13(4):345–352. doi: 10.1016/S1470-2045(11)70373-4.
- Tokunaga E, Okada S, Yamashita N, et al. High incidence and frequency of LOH are associated with aggressive features of high-grade HER2 and triple-negative breast cancers. Breast Cancer. 2012;19(2):161–169. doi: 10.1007/s12282-010-0232-7.
- Kikuchi S, Nishimura R, Osako T, et al. Definition of p53 overexpression and its association with the clinicopathological features in luminal/HER2-negative breast cancer. Anticancer Res. 2013;33:3891–3897.
- Satoh K, Tanaka M, Yano A, et al. Treatment when prognostic factors do not match St. Gallen recommendations: profiling of prognostic factors among HR(+) and HER2(–) breast cancer patients. World J Surg. 2013;37(3):516–524. doi: 10.1007/s00268-012-1881-9.
- Kurebayashi J, Kanomata N, Shimo T, et al. Marked lymphovascular invasion, progesterone receptor negativity, and high Ki67 labeling index predict poor outcome in breast cancer patients treated with endocrine therapy alone. Breast Cancer. 2014;21(2):214–222. doi: 10.1007/s12282-012-0380-z.
- Niikura N, Masuda S, Kumaki N, et al. Prognostic significance of the Ki67 scoring categories in breast cancer subgroups. Clin Breast Cancer. 2014;14(5):323–329.e3. doi: 10.1016/j.clbc.2013.12.013.
- Nishimiya H, Kosaka Y, Yamashita K, et al. Prognostic significance of Ki-67 in chemotherapy-naïve breast cancer patients with 10-year follow-up. Anticancer Res. 2014;34:259–268.
- Hisamatsu Y, Tokunaga E, Yamashita N, et al. Impact of GATA-3 and FOXA1 expression in patients with hormone receptor-positive/HER2-negative breast cancer. Breast Cancer. 2015;22(5):520–528. doi: 10.1007/s12282-013-0515-x.
- Honma N, Horii R, Ito Y, et al. Differences in clinical importance of bcl-2 in breast cancer according to hormone receptors status or adjuvant endocrine therapy. BMC Cancer. 2015;15(1):698. doi: 10.1186/s12885-015-1686-y.
- Kawase M, Toyama T, Takahashi S, et al. FOXA1 expression after neoadjuvant chemotherapy is a prognostic marker in estrogen receptor-positive breast cancer. Breast Cancer. 2015;22(3):308–316. doi: 10.1007/s12282-013-0482-2.
- Nishimukai A, Yagi T, Yanai A, et al. High Ki-67 expression and low progesterone receptor expression could independently lead to a worse prognosis for postmenopausal patients with estrogen receptor-positive and HER2-negative breast cancer. Clin Breast Cancer. 2015;15(3):204–211. doi: 10.1016/j.clbc.2014.12.007.
- Ono M, Tsuda H, Yunokawa M, et al. Prognostic impact of Ki-67 labeling indices with 3 different cutoff values, histological grade, and nuclear grade in hormone-receptor-positive, HER2-negative, node-negative invasive breast cancers. Breast Cancer. 2015;22(2):141–152. doi: 10.1007/s12282-013-0464-4.
- Higuchi T, Nishimukai A, Ozawa H, et al. Prognostic significance of preoperative 18F-FDG PET/CT for breast cancer subtypes. Breast. 2016;30:5–12. doi: 10.1016/j.breast.2016.08.003.
- Kawai M, Tomotaki A, Miyata H, et al. Body mass index and survival after diagnosis of invasive breast cancer: a study based on the Japanese national clinical database–breast cancer registry. Cancer Med. 2016;5(6):1328–1340. doi: 10.1002/cam4.678.
- Miyoshi Y, Shien T, Ogiya A, et al. Differences in expression of the cancer stem cell marker aldehyde dehydrogenase 1 among estrogen receptor-positive/human epidermal growth factor receptor type 2-negative breast cancer cases with early, late, and no recurrence. Breast Cancer Res. 2016;18(1):73. doi: 10.1186/s13058-016-0731-3.
- Okuma HS, Koizumi F, Hirakawa A, et al. Clinical and microarray analysis of breast cancers of all subtypes from two prospective preoperative chemotherapy studies. Br J Cancer. 2016;115(4):411–419. doi: 10.1038/bjc.2016.184.
- Sato K, Miyashita M, Ishida T, et al. Prognostic significance of the progesterone receptor status in Ki67-high and low luminal B-like HER2-negative breast cancers. Breast Cancer. 2016;23(2):310–317. doi: 10.1007/s12282-014-0575-6.
- Kurozumi S, Matsumoto H, Hayashi Y, et al. Power of PgR expression as a prognostic factor for ER-positive/HER2-negative breast cancer patients at intermediate risk classified by the Ki67 labeling index. BMC Cancer. 2017;17(1):354. doi: 10.1186/s12885-017-3331-4.
- Ono M, Tsuda H, Yoshida M, et al. Prognostic significance of progesterone receptor expression in estrogen-receptor positive, HER2-negative, node-negative invasive breast cancer with a low Ki-67 labeling index. Clin Breast Cancer. 2017;17(1):41–47. doi: 10.1016/j.clbc.2016.06.012.
- Hayashi N, Takahashi Y, Matsuda N, et al. The prognostic effect of changes in tumor stage and nodal status after neoadjuvant chemotherapy in each primary breast cancer subtype. Clin Breast Cancer. 2018;18(2):e219–29–e229. doi: 10.1016/j.clbc.2017.09.013.
- Imamura M, Morimoto T, Nomura T, et al. Independent prognostic impact of preoperative serum carcinoembryonic antigen and cancer antigen 15-3 levels for early breast cancer subtypes. World J Surg Oncol. 2018;16(1):26. doi: 10.1186/s12957-018-1325-6.
- Kurozumi S, Matsumoto H, Inoue K, et al. Impact of combining the progesterone receptor and preoperative endocrine prognostic index (PEPI) as a prognostic factor after neoadjuvant endocrine therapy using aromatase inhibitors in postmenopausal ER positive and HER2 negative breast cancer. PLoS One. 2018;13(8):e0201846. doi: 10.1371/journal.pone.0201846.
- Yamada Y, Mukai H, Tokudome Y, et al. Improved overall survival over recent decades in patients with hormone-receptor-positive, HER2-negative breast cancer: a single-center retrospective analysis of prognostic factors. Jpn J Clin Oncol. 2018;48(3):248–254. doi: 10.1093/jjco/hyy001.
- Arima N, Nishimura R, Osako T, et al. Ki-67 index value and progesterone receptor status can predict prognosis and suitable treatment in node-negative breast cancer patients with estrogen receptor-positive and HER2-negative tumors. Oncol Lett. 2019;17:616–622.
- Fujimoto Y, Watanabe T, Hida AI, et al. Prognostic significance of tumor-infiltrating lymphocytes may differ depending on Ki67 expression levels in estrogen receptor-positive/HER2-negative operated breast cancers. Breast Cancer. 2019;26(6):738–747. doi: 10.1007/s12282-019-00977-0.
- Naoi Y, Saito Y, Kishi K, et al. Development of recurrence risk score using 95‑gene classifier and its application to formalin‑fixed paraffin‑embedded tissues in ER‑positive, HER2‑negative and node‑negative breast cancer. Oncol Rep. 2019;42(6):2680–2685. doi: 10.3892/or.2019.7358.
- Ohara AM, Naoi Y, Shimazu K, et al. PAM50 for prediction of response to neoadjuvant chemotherapy for ER-positive breast cancer. Breast Cancer Res Treat. 2019;173(3):533–543. doi: 10.1007/s10549-018-5020-7.
- Takuwa H, Tsuji W, Yotsumoto F, et al. Prevention of locoregional recurrence and distant metastasis in japanese breast cancer patients using Japanese standard postoperative radiation fields: experience at a single institution. Cancer Rep. 2019;2:e1191.
- Horimoto Y, Sasahara N, Sasaki R, et al. High FOXA1 protein expression might predict late recurrence in patients with estrogen-positive and HER2-negative breast cancer. Breast Cancer Res Treat. 2020;183(1):41–48. doi: 10.1007/s10549-020-05751-x.
- Ishiguro H, Masuda N, Sato N, et al. A randomized study comparing docetaxel/cyclophosphamide (TC), 5-fluorouracil/epirubicin/cyclophosphamide (FEC) followed by TC, and TC followed by FEC for patients with hormone receptor-positive HER2-negative primary breast cancer. Breast Cancer Res Treat. 2020;180(3):715–724. doi: 10.1007/s10549-020-05590-w.
- Sugano T, Yoshida M, Masuda M, et al. Prognostic impact of ACTN4 gene copy number alteration in hormone receptor-positive, HER2-negative, node-negative invasive breast carcinoma. Br J Cancer. 2020;122(12):1811–1817. doi: 10.1038/s41416-020-0821-y.
- Fujihara M, Yamasaki R, Ito M, et al. Risk factors of local recurrence following implant-based breast reconstruction in breast cancer patients. BMC Womens Health. 2021;21:147.
- Takahashi S, Fukui T, Nomizu T, et al. TP53 signature diagnostic system using multiplex reverse transcription–polymerase chain reaction system enables prediction of prognosis of breast cancer patients. Breast Cancer. 2021;28(6):1225–1234. doi: 10.1007/s12282-021-01250-z.
- Tanaka Y, Ohno T, Kadonaga T, et al. Podoplanin expression in cancer-associated fibroblasts predicts unfavorable prognosis in node-negative breast cancer patients with hormone receptor-positive/HER2 − negative subtype. Breast Cancer. 2021;28(4):822–828. doi: 10.1007/s12282-021-01217-0.
- Toi M, Imoto S, Ishida T, et al. Adjuvant S-1 plus endocrine therapy for oestrogen receptor-positive, HER2-negative, primary breast cancer: a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021;22(1):74–84. doi: 10.1016/S1470-2045(20)30534-9.
- Kurozumi S, Kaira K, Matsumoto H, et al. Association of L-type amino acid transporter 1 (LAT1) with the immune system and prognosis in invasive breast cancer. Sci Rep. 2022;12(1):2742. doi: 10.1038/s41598-022-06615-8.
- Sueta A, Yamamoto-Ibusuki M, Tomiguchi M, et al. Predictive and prognostic significance of BRCAness in HER2-negative breast cancer. Breast Cancer. 2022;29(2):368–376. doi: 10.1007/s12282-021-01319-9.
- Yamamoto S, Chishima T, Shibata Y, et al. Stratification of prognosis by biological features following neoadjuvant chemotherapy in luminal breast cancer. In Vivo. 2022;36(2):859–864. doi: 10.21873/invivo.12774.
- Burstein HJ, Curigliano G, Thürlimann B, et al. Customizing local and systemic therapies for women with early breast cancer: the St. Gallen international consensus guidelines for treatment of early breast cancer 2021. Ann Oncol. 2021;32(10):1216–1235. doi: 10.1016/j.annonc.2021.06.023.
- Johnston SRD, Harbeck N, Hegg R, et al. Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2−, node-positive, high-risk, early breast cancer (monarchE). J Clin Oncol. 2020;38(34):3987–3998. doi: 10.1200/JCO.20.02514.
- Allison KH, Hammond MEH, Dowsett M, et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J Clin Oncol. 2020;38(12):1346–1366. doi: 10.1200/JCO.19.02309.
- Beadle BM, Woodward WA, Buchholz TA. The impact of age on outcome in early-stage breast cancer. Semin Radiat Oncol. 2011;21(1):26–34. doi: 10.1016/j.semradonc.2010.09.001.