Abstract
Australia and New Guinea have experienced episodic connection and separation by high sea levels since the early Miocene. This has markedly influenced biotic patterns, although exactly how remains puzzling in some instances. One example concerns the palaeozoogeography of forest wallabies (Dorcopsini), all six living species of which are found only in New Guinea, even though most dorcopsin fossils recognized are from Australia. Here we review the taxonomic identity of ‘Silvaroo’ buloloensis from the late Pliocene Otibanda Formation of eastern Papua New Guinea, and show that, according to dental evidence, it is a dorcopsin along with its contemporary, Watutia novaeguineae. Phylogenetic analysis reconstructs ‘S.’ buloloensis as sister to Dorcopsoides fossilis from the late Miocene of central Australia. The degree of dental similarity between them leads us to include it in the same genus. These findings build upon other recent research on extinct New Guinea macropodines, revealing that both forest wallabies and tree-kangaroos are better represented in the fossil record than previously believed, although intriguingly none are ascribable to modern genera.
Isaac A. R. Kerr [[email protected]], College of Science and Engineering, Flinders University, Bedford Park, South Australia 5042, Australia. Gavin J. Prideaux [[email protected]], College of Science and Engineering, Flinders University, Bedford Park, South Australia 5042, Australia.
KANGAROOS, wallabies and their kin (family Macropodidae) are common herbivorous marsupials found across almost all terrestrial ecosystems in Australia and New Guinea (Grigg et al. Citation1989, Johnson et al. Citation1989). The evolutionary origins of macropodids lie in the rainforests of late Oligocene–early Miocene Australia, where small, possum-like, probably quadrupedal macropodids fed chiefly on leaves, fruits and fungi in dense vegetation (Long et al. Citation2002, Black et al. Citation2012, Janis et al. Citation2016). Much of the research on macropodid evolution focuses on the profound effects of the late Miocene Australian aridification and mid-Pliocene spread of grasslands on the development of larger body size, grazing-adapted crania and dentition, and derived methods of locomotion such as efficient bipedal saltation (e.g., Helgen et al. Citation2006, Kear et al. Citation2008, Janis et al. Citation2014, Couzens & Prideaux Citation2018, Janis et al. Citation2023). However, the macropodid identity is considerably more diverse than this, and groups of smaller, less well-studied macropodines, both fossil and living, exhibit intriguing glimpses into the palaeohistory of kangaroos and wallabies (e.g., Groves & Flannery Citation1989, Prideaux & Warburton Citation2023).
New Guinea was sporadically linked to mainland Australia by a land-bridge during periods of low sea level as recently as the last glacial maximum and as far back as the early Miocene, facilitating faunal exchange across the Torres Strait (Walker Citation1972, Flannery Citation1990a, Macqueen et al. Citation2010, Beck Citation2017). Though the fossil record in New Guinea and southeastern Indonesia is poorly known, especially prior to the mid-Pliocene (Black et al. Citation2012), phylogenetic studies of modern-day marsupials in these regions have suggested that marsupials of living families dispersed into New Guinea from Australia at least during the early Miocene (Aplin et al. Citation1993, Beck Citation2017). Since New Guinea did not undergo aridification in late Miocene–Pleistocene, and with the dramatic uplift of the New Guinean highlands from 8 to 4 Ma (Cloos et al. Citation2005, van Ufford & Cloos Citation2005) providing extensive rainforest habitat, New Guinea remained hospitable to forest-adapted marsupials (Macqueen et al. Citation2011, Black et al. Citation2012). As recent research has suggested, the Pliocene and Pleistocene macropodid fauna of New Guinea thus contains a significant number of basal macropodines (Kerr & Prideaux Citation2022, Prideaux & Warburton Citation2023).
Extant basal macropodines are much better represented in New Guinea than in mainland Australia. Members of the crown tribe Macropodini have few occurrences in New Guinea despite being extremely speciose within Australia (Helgen Citation2007, Jackson & Groves Citation2015). The six extant species of Dorcopsulus Matschie, Citation1916 and Dorcopsis Quoy & Gaimard, Citation1830a, the only living members of Dorcopsini, the most plesiomorphic macropodine tribe (Prideaux & Warburton Citation2010, Cascini et al. Citation2019), are endemic to New Guinea and its neighbouring islands (Flannery Citation1990b; Helgen Citation2007). Four of the seven living species of Thylogale Gray, Citation1837 (the pademelons), a genus either sister to tribe Dendrolagini or a basal lineage within the tribe (Cascini et al. Citation2019, Beck et al. Citation2022, Westerman et al. Citation2022, Prideaux & Warburton Citation2023), are today endemic to New Guinea, with another species found in both southeastern New Guinea and eastern Australia (Flannery Citation1990b, Helgen Citation2007, Macqueen et al. Citation2010). The living tree-kangaroos (species of Dendrolagus Müller, Citation1840) are also most diverse in New Guinea (Flannery Citation1990b, Jackson & Groves Citation2015, Eldridge et al. Citation2018).
Despite the present-day diversity of plesiomorphic macropodines in New Guinea, the fossil record of dorcopsins and dendrolagins overwhelmingly derives from the Australian mainland. The dorcopsin fossil record is scarce, with only three fossil species known: Dorcopsoides fossilis Woodburne, Citation1967 from the late Miocene Alcoota locality in central Australia (); Dorcopsis wintercookorum Flannery, Rich, Turnbull & Lundelius, 1992 from the early Pliocene Hamilton Local Fauna in western Victoria; and Watutia novaeguineae Flannery, Hoch & Aplin, Citation1989 from the late Pliocene Otibanda Formation in eastern Papua New Guinea (). Watutia novaeguineae was recently considered a dorcopsin by Prideaux & Warburton (Citation2023) on the basis of dental character states, particularly of the third premolar. Dendrolagins are better represented, with seven fossil species recognized, all of which are placed within the genus Bohra Flannery & Szalay, Citation1982 (Prideaux & Warburton Citation2023). Only one of these is known from New Guinea—Bohra planei Prideaux & Warburton, Citation2023, found in the Otibanda Formation. This species was recently described from a partial hindlimb, previously considered by Plane (Citation1967) to possibly represent a species of Dorcopsis. This imbalance of fossil species may be due to the longer history of exploration in Australian fossil deposits compared to those from New Guinea.
Fig. 1. Map of significant fossil localities mentioned in the text with epoch designated by symbol. LF = local fauna.
When Woodburne (Citation1967) described D. fossilis he considered it to belong to the subfamily Potoroinae, along with the species of Dorcopsis and Dorcopsulus. However, the latter are today grouped in the basal macropodine tribe Dorcopsini on the basis of dental and osteological characters (Prideaux & Warburton Citation2010, Beck et al. Citation2022), and supported by molecular data in the case of the living genera (Cascini et al. Citation2019, Westerman et al. Citation2022). Per Prideaux & Warburton (Citation2010), a single unambiguous synapomorphy links the dorcopsins: lateral constriction of the upper permanent premolar (P3) immediately anterior to the posterolingual cusp. Dorcopsis and Dorcopsulus are considered the most basal extant macropodine genera (Cascini et al. Citation2019, Westerman et al. Citation2022). These genera estimated to have diverged from Dorcopsoides Woodburne, Citation1967 by at least 10 Ma (Burk et al. Citation1998, Cascini et al. Citation2019).
One New Guinean fossil taxon, ‘Silvaroo’ buloloensis (Plane Citation1967) from the Otibanda Formation, may represent an unrecognized New Guinean fossil dorcopsin. Though formerly part of the macropodine genus Protemnodon Owen, Citation1874, the tribe and genus of ‘S.’ buloloensis are currently indeterminate (Prideaux & Warburton Citation2023, p. 42). This follows the recognition by Prideaux & Warburton (Citation2023) that two of the three species of Silvaroo Dawson, Citation2004, including the type species Silvaroo bila Dawson, Citation2004, belong in the dendrolagin genus Bohra, an extinct group of large tree-kangaroos. Silvaroo was thus rendered a junior synonym of Bohra. Flannery et al. (Citation1989) discussed features of ‘Protemnodon’ buloloensis that suggested affinities with species of Dorcopsis more than with those of Protemnodon, highlighting the narrow, gently curved premolars and the number and position of their ridgelets. However, Flannery et al. (Citation1989) left ‘P.’ buloloensis within Protemnodon due to taxonomic uncertainty, until additional material and improved understanding of modern groups facilitates its re-examination. When describing Silvaroo and allocating ‘P.’ buloloensis to it, Dawson (Citation2004) demonstrated that ‘S.’ buloloensis bears phenetic similarities to the dorcopsin genera Dorcopsis and Dorcopsulus, but did not put forward a phylogenetic hypothesis. There is thus considerable confusion regarding the affinities of ‘S.’ buloloensis, and good reason to believe that it may constitute an unrecognized relative of the living dorcopsins of New Guinea.
The generic identity of ‘S.’ buloloensis clearly needs clarification, which we will undertake herein by comparing and re-diagnosing the taxon. Improved understanding of the relationships of macropodines both living and fossil since the late 1980s, particularly with the erection of tribe Dorcopsini, provides greater clarity than was available in previous studies. We also seek to make clear the evolutionary relationships of ‘S.’ buloloensis and W. novaeguineae to other macropodids by placing them both in a parsimony analysis focused on stem macropodids, and the bases of the subfamilies Sthenurinae and Macropodinae.
Materials and methods
Nomenclature
Dental numeration follows Flower (Citation1867) and Luckett (Citation1993). Dental and osteological nomenclature follows Prideaux (Citation2004) and Kerr & Prideaux (Citation2022).
Institutional abbreviations
ANU, Australian National University, Canberra, Australia; ANWC CM, Australian National Wildlife Collection, Canberra, Australia; CPC, Commonwealth Palaeontology Collection, Geoscience Australia, Canberra, Australia; FUR, Vertebrate Collection, Flinders University, Adelaide, Australia; NHMD, Natural History Museum of Denmark, Copenhagen, Denmark; NMV, Museums Victoria, Melbourne, Australia; PNG, Papua New Guinea National Museum and Art Gallery, Port Moresby, Papua New Guinea; QM, Queensland Museum, Brisbane, Australia; SAMA, South Australian Museum, Adelaide, Australia; UCMP, University of California Museum of Paleontology, Berkeley, CA, USA; WAM, Western Australian Museum, Perth, Australia.
Comparative material
Digitized specimens were accessed via OzBoneViz (https://sketchfab.com/Ozboneviz) and MorphoSource (www.morphosource.org). A table of specimens used in the taxonomic and phylogenetic analyses and their provenance is included in Supplementary Data 1.
We compared material referred to ‘Silvaroo’ buloloensis with specimens of all extant dorcopsins: Dorcopsis luctuosa (D’Albertis, Citation1874), Dorcopsis atrata van Deusen, Citation1957, Dorcopsis muelleri (Lesson, Citation1827), Dorcopsis hageni Heller, Citation1897, Dorcopsulus vanheurni (Thomas, Citation1922) and Dorcopsulus macleayi (De Miklouho-Maclay, Citation1885); and the fossil dorcopsins Dorcopsis wintercookorum, Dorcopsoides fossilis and Watutia novaeguineae. We extended comparisons to the macropodin Protemnodon otibandus and the dendrolagin Bohra planei, because ‘S.’ buloloensis has previously been allied with these taxa, and both are known from the Otibanda Formation (Plane Citation1967, Dawson Citation2004, Prideaux & Warburton Citation2023). Bohra bandharr (Dawson, Muirhead & Rowe, Citation1999) and Bohra bila (Dawson, Citation2004) were also included because both were previously placed in Silvaroo (Dawson Citation2004), and because the P3 and dentary of B. planei are not known (Prideaux & Warburton Citation2023).
Other living comparative taxa included: Dendrolagus lumholtzi Collett, Citation1884, Setonix brachyurus (Quoy & Gaimard, Citation1830b), Thylogale billardierii (Desmarest, Citation1822), Wallabia bicolor (Desmarest, Citation1804) and Petrogale xanthopus Gray, Citation1855. These are members of key basal macropodine genera, with the exception of W. bicolor, which is included as a basal member of the crown tribe Macropodini (Cascini et al. Citation2019, Westerman et al. Citation2022). Non-macropodine fossil macropodids included Hadronomas puckridgi Woodburne, Citation1967, Wanburoo hilarus Cooke, Citation1999, Wabularoo prideauxi Travouillon, Archer & Hand, Citation2015, Cookeroo hortusensis Butler, Travouillon, Price, Archer & Hand, Citation2016 and Ganguroo robustiter Cooke, Travouillon, Archer & Hand, Citation2015. Wanburoo hilarus and W. prideauxi were included as they are considered early members of subfamily Sthenurinae (Travouillon et al. Citation2015, Travouillon et al. Citation2022), and H. puckridgi and W. hilarus have been described as morphologically similar to W. novaeguineae (Flannery et al. Citation1989). Cookeroo hortusensis and G. robustiter were included as they are the more derived of the various basal macropodids and both their upper and lower dentitions are known (Cooke et al. Citation2015, Butler et al. Citation2016).
Phylogenetic analysis
A maximum parsimony analysis was carried out with the aim of testing the conclusions of the taxonomic analysis with regards to the affinities of ‘Silvaroo’ buloloensis. The matrix of 120 morphological characters (47 dental, 73 osteological) was adapted from Travouillon et al. (Citation2022), which derived from Kear & Pledge (Citation2007) and Prideaux & Warburton (Citation2010), with additional characters modified from Prideaux (Citation2004) and Prideaux & Warburton (Citation2023). Original characters, created via observation of basal macropodine material were added to target Dorcopsini and clarify the relationships within the tribe. The complete character matrix is available in Supplementary Data 2.
Characters were scored for 65 macropodoid species. The extant Hypsiprymnodon moschatus Ramsay, Citation1875 (family Hypsiprymnodontidae) was selected as the outgroup in compliance with other recent phylogenetic analyses of Macropodinae (e.g., Travouillon et al. Citation2015, Cascini et al. Citation2019, Westerman et al. Citation2022, Prideaux & Warburton Citation2023). To help establish ancestral characteristics, we included a propleopine (Ekaltadeta ima Archer & Flannery, Citation1985) and five potoroids: Aepyprymnus rufescens (Gray, Citation1837), Potorous tridactylus (Kerr, Citation1792), Bettongia penicillata Gray, Citation1837, Bettongia lesueur (Quoy & Gaimard, Citation1824), and Wakiewakie lawsoni (Woodburne, Citation1984). Within Macropodidae, taxa were selected with the aim of best representing basal macropodids and the divergence of Lagostrophinae, Sthenurinae and Macropodinae. Basal macropodids from five genera were chosen, with a preference given to those represented by complete dentitions and described postcranial material: Ngamaroo archeri Kear & Pledge, Citation2007, Gumardee webbi Travouillon, Butler, Archer & Hand, Citation2022, Gumardee keari Travouillon, Butler, Archer & Hand, Citation2022, Bulungamaya delicata Flannery, Archer & Plane, Citation1983, Ganguroo bilamina Cooke, Citation1997, Ganguroo robustiter, and Cookeroo hortusensis. Three species of lagostrophine were scored: Lagostrophus fasciatus (Péron & Lesueur, Citation1807), Troposodon minor (Owen, Citation1877a) and Tjukuru wellsi Prideaux & Tedford, Citation2012. Species from five key sthenurine genera were scored: Hadronomas puckridgi, Rhizosthenurus flanneryi Kear, Citation2002, Archaeosimos cegsai Prideaux, Citation2004, Metasthenurus newtonae (Prideaux, Citation2000), and Sthenurus andersoni Marcus, Citation1962); in addition, the species of Wanburoo Cooke, Citation1999 and Wabularoo Archer, Citation1979 are probably basal sthenurines similar to ‘S.’ buloloensis and W. novaeguineae (Prideaux Citation2004, Travouillon et al. Citation2015). From within Macropodinae, all living dorcopsins were scored, though in the case of Dorcopsulus macleayi and Dorcopsis hageni, only craniodental material could be sourced. The early Pliocene Dorcopsis wintercookorum, known from fragmentary dental remains, was also included. Genera that were previously thought to contain ‘S.’ buloloensis were sampled more completely: four species each of Protemnodon and Bohra. In total, species from 20 macropodine genera were scored, with representation from key extant and fossil taxa. In addition, the basal macropodine Nombe nombe from the Pleistocene of Papua New Guinea was included, because it is not currently affiliated with any of the three macropodine tribes.
We undertook a parsimony analysis using a TBR search in command-line TNT v3.81 (Goloboff et al. Citation2008): 10,000 trees held, 10,000 replications, 10 starting trees for each replication. Tree length, tree scores, bootstrapping and jackknifing values were generated. All characters were unweighted and unordered. The included lagostrophines, L. fasciatus, T. minor and T. wellsi were constrained outside Macropodinae because we considered them convergent on macropodines based on previous studies (Prideaux & Warburton Citation2010, Butler et al. Citation2016, Cascini et al. Citation2019, Westerman et al. Citation2022). Most parsimonious trees and a strict consensus tree were transferred to the tree-editing program FigTree v1.4.4 and then to Adobe Illustrator v28 for editing and formatting.
Systematic palaeontology
Class MAMMALIA Linnaeus, Citation1758
Order DIPROTODONTIA Owen, Citation1877b
Suborder MACROPODIFORMES Ameghino, Citation1889
Superfamily MACROPODOIDEA Gray, Citation1821
Family MACROPODIDAE Gray, Citation1821
Subfamily MACROPODINAE Gray, Citation1821
Tribe DORCOPSINI Prideaux & Warburton, Citation2010
Dorcopsoides Woodburne, Citation1967
Type species
Dorcopsoides fossilis Woodburne, Citation1967.
Diagnosis
The two species of Dorcopsoides share a unique combination of craniodental traits that exclude them from all other macropodine taxa. Dorcopsoides is most similar to Dorcopsis, Dorcopsulus, Watutia and Setonix in dental characteristics, with no single trait distinguishing all similar genera. Dorcopsoides is differentiated from Dorcopsulus, Watutia and Setonix by having a postcingulid on its lower molars. Dorcopsoides differs from Dorcopsis and Dorcopsulus in having a shorter P3 relative to M1, with fewer cuspules, and lacking anterolingual curvature in occlusal view; and p3 lacking a gently anterolingually curved main crest. Dorcopsoides further differs from Dorcopsulus in having a dentary with a distinct posterior mental foramen; P3 with a slight lingual cingulum; p3 with a more distinct waist immediately anterior to the posterior cuspule; and lower molars with a more anteriorly prominent paracristid and a less buccally situated cristid obliqua. Dorcopsoides is further differentiated from Watutia in having P3 and p3 with a larger, more anteriorly prominent anterior cuspule and less raised ridgelets between the anterior-most and posterior-most cuspules; P3 with a slight lingual cingulum; upper molars with a more anteriorly prominent precingulum; and lower molars with a lower, less distinct premetacristid. Dorcopsoides additionally differs from Setonix in having a dentary with a less prominent digastric eminence; more elongate P3 and p3 relative to width, with a distinct waist anterior to the posterior cuspule; P3 with a lower, narrower lingual cingulum, and a more elongate buccal cingulum; and upper molars with a more prominent stylar cusp C.
Etymology
Named for its similarity to species of Dorcopsis.
Dorcopsoides buloloensis (Plane, Citation1967)
(, ; )
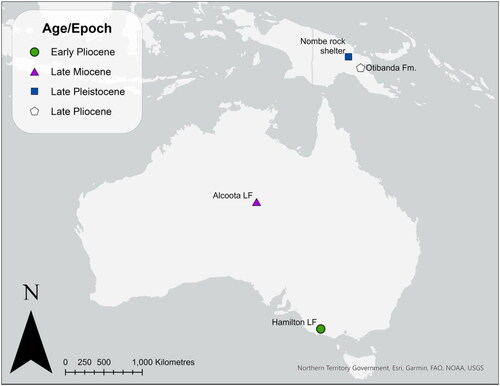
Fig. 2. Left P3–M4 of A, Dorcopsis luctuosa specimen ANWC CM3646 and B, Dorcopsoides fossilis specimen ‘ASP8810’, in occlusal view; C–E, left P3–M3 of Dorcopsoides buloloensis referred specimen NHMD 193273 in occlusal, lingual and buccal views; F–H, left I1 in anterior, medial and lateral views; and I–K, right M4 in occlusal, lingual and buccal views; L–N, left M3 of D. buloloensis referred specimen NHMD 193282 in occlusal, buccal and lingual views; and O–Q, left P3–M4 of Watutia novaeguineae holotype in occlusal, lingual and buccal views.
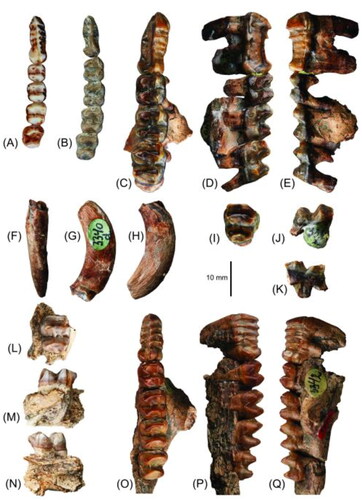
Fig. 3. Left p3–m4 of A, Dorcopsis luctuosa specimen C M3646 and B, Dorcopsoides fossilis specimen FU 0356, in occlusal view; C–E, left p3 and m2–4 of Dorcopsoides buloloensis referred specimen NHMD 193280 in occlusal, buccal and lingual views; F–H, M2 and partial M3; and I–K, right i1 of D. buloloensis referred specimen NHMD 193282 in occlusal, lingual and buccal views.
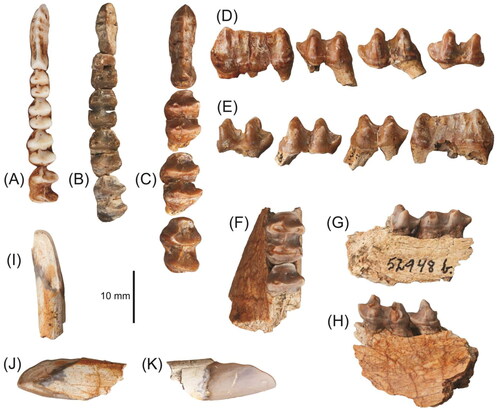
Table 1. Dimensions in millimetres of permanent upper cheek teeth of specimens of Dorcopsoides buloloensis and Dorcopsoides fossilis.
Table 2. Dimensions in millimetres of permanent lower cheek teeth of specimens of Dorcopsoides buloloensis and Dorcopsoides fossilis.
1967, Protemnodon buloloensis Plane, pp. 44–47, fig. 11.
1989, Protemnodon buloloensis, Flannery et al., pp. 150–151, fig. 1A–C, 1I, table 2.
2004, Silvaroo buloloensis Dawson, p. 284, 286, table 1.
2023, ‘Silvaroo’ buloloensis Prideaux & Warburton, p. 65.
Diagnosis
Dorcopsoides buloloensis differs from Dorcopsoides fossilis in its larger size and in having a dentary with a shorter diastema, a more posteriorly situated anterior mental foramen, and a small but distinct posterior mental foramen; and a more robust i1 with a thicker, less raised ventrolingual crest.
Etymology
Named for Bulolo, a nearby town to the southeast of the type locality.
Holotype
CPC 6774 (UCMP 45243), anterior portion of adult left dentary with moderately worn i1, p3, m1–2 in place and loose partial m3 and m4. Anterior two-thirds of dentary below diastema and mandibular corpus posterior to m2 not preserved.
Referred material
NHMD 193282 (previously GMK 52948), a partial left M2, left M3 in maxillary fragment, right i1, partial m1, right m2, and left m2 and partial m3 in dentary fragment; NHMD 193280 (previously GMK 55708), left p3 and m2–4. NHMD 193273 (previously GMK 52956), a left I1 with root and base of crown, left and right P3, left M1–3 in maxillary fragment, right M3 in maxillary fragment, and right M4. NHMD 193278 (previously GMK 55173), partial right dentary with p3–m4, proximal diastema and anterior base of ascending ramus.
Type locality, unit and age
‘Sunshine General’ (UCMP V5564), at the Sunshine alluvial gold sluicing workings in the type section of the Otibanda Formation, near Bulolo, Papua New Guinea. Potassium–argon dating of a sample of pyroclastic rock from an outcrop of the Otibanda Formation close to Watut 3 locality on the eastern bank of the Watut river returned an age of 2.9 ± 0.4 Ma (Hoch & Holm Citation1986), though this cannot be directly related to the Sunshine General locality (Prideaux & Warburton Citation2023), which is therefore considered to be broadly of late Pliocene age. Referred material is derived from the Otibanda Formation at Widubosh goldmine (NHMD 193282, NHMD 193280), Watut River near Awe Village (NHMD 193273), and Mainyanda (NHMD 193278).
Description and comparisons
Upper dentition
The only known I1 of Dorcopsoides buloloensis (NHMD 193273) preserves the base of the enamel crown and the root (). The crown is fairly narrow and oval, with the posteriorly curved root becoming increasingly transversely compressed towards its base. The only two known P3s (NHMD 193273) are moderately and heavily worn, removing much of the lingual morphology (). The P3 is narrow and elongate, narrowing anteriorly, with a distinct waist immediately anterior to the posterior cuspule. The high, sectorial main crest is composed of six cuspules and extends anteriorly through the anterior-most cuspule up the anterior surface to the base of the crown. In occlusal view the crest is straight in its anterior portion, curving gently buccally to the posterior cuspule, which is slightly buccally offset. In buccal view the crest is saddle-shaped when moderately worn. The anterior cuspule is the most raised, with a strongly convex, curved anterior margin in buccal view. Narrow, raised buccal ridgelets extend dorsally from all but the posterior cuspule; the ridgelet from the anterior cuspule is most raised and distinct, while that of the fourth cuspule is very fine and slight. The lingual ridgelets have been almost entirely removed by wear, present as slight swellings at the base of each cuspule bar the posterior cuspule. The lingual cingulum is very low and does not form a distinct crest but rather a thick lingual swelling with small peaks at the base of the third and fourth cuspules, extending from the posterolingual cuspule to the base of the second cuspule. The posterolingual cuspule is moderately worn; it is situated lingual to the more raised posterior cuspule. A thick transverse crest extends lingually and slightly posteriorly from immediately anterior to the posterior cuspule to the posterolingual cuspule. A buccal cingulum is present as a slight swelling at the base of the buccal enamel extending the length of the P3.
The upper molars are known from a specimen preserving left M1–M3 and right M3–M4 (NHMD 193273) and one with a partial M2 and M3 (NHMD 193282), all moderately worn such that dentine is visible on all but one loph of one M3 (, I–N). The upper molars are low crowned, rounded-rectangular, and fairly short and broad. Absolute length and loph widths increase to M3 then decrease in M4. Protoloph and metaloph width are subequal in M1 and M2; the protoloph is relatively much wider than the metaloph in M3 and M4. Both loph crests are gently anteriorly convex in occlusal view. Stylar cusp C is present in M1 as a small, narrow crest at the posterobuccal base of the protoloph, and is present as a swelling in M2 and M3. Stylar cusp D is similarly present in M1 as a small narrow crest at the anterobuccal base of the metaloph, and is present as a small swelling in M2. The precingulum is short with a straight anterior margin and a slightly rounded corner at the buccal margin where it merges with the thin but distinct preparacrista. A slight swelling at the midpoint of the precingulum of the M3 of NHMD 193282 suggests the presence of a preprotocrista (forelink) removed by wear. The postparacrista is thin but distinct, extending posteriorly from the paracone and curving slightly lingually into the interloph valley. The postprotocrista is thick and low, curving buccally and posteriorly from the protocone, then continuing posteriorly into the interloph valley. A thick, low premetaconulecrista is present in the interloph valley, but is otherwise removed by wear. The thin but distinct premetacrista extends posteriorly and slightly buccally from the base of the postparacrista to the metacone. The thick, raised postmetaconulecrista extends posteriorly and buccally from the metaconule, curving beneath and posteriorly bounding a small, deep posterior basin. The postmetacrista is short and thin, extending straight posteriorly along the buccal margin of the posterior basin to the buccal end of the postmetaconulecrista. The molars are too worn for the presence of a urocrista to be ascertained.
The base of the crown of I1 of D. buloloensis is slightly broader relative to length than that of Wanburoo hilarus and Ganguroo robustiter, and slightly narrower relative to length than that of Wallabia bicolor, Thylogale billardierii and Petrogale xanthopus. It is otherwise too incomplete for meaningful comparisons.
The P3 of D. buloloensis is very similar to that of Dorcopsoides fossilis. It is also very similar to that of the species of Dorcopsis and Dorcopsulus, but differs in being shorter relative to the M1, having fewer cuspules, and lacking a slight anterolingual curvature in occlusal view. It further differs from that of Dorcopsulus vanheurni and Du. macleayi in having a slightly broader lingual cingulum with more prominent cuspules, and additionally from D. vanheurni in having a narrower anterior cuspule relative to the posterior. The P3 is distinguished from that of Watutia novaeguineae () by having a larger, more anteriorly prominent anterior cuspule, less raised ridgelets from the second, third and fourth cuspules, and a slight lingual cingulum. It differs from that of all compared taxa except the species of Dorcopsis and Dorcopsulus, W. novaeguineae and D. fossilis in having a distinct waist anterior to the posterior cuspule, and is differentiated from all compared macropodins and dendrolagins bar Bohra bandharr, Dendrolagus lumholtzi and Setonix brachyurus in having a very slight buccal cingulum. The P3 differs from that of Protemnodon otibandus, W. bicolor, S. brachyurus, B. bandharr, D. lumholtzi, T. billardierii and Hadronomas puckridgi in having a lower (i.e., not raised into a low crest), narrower lingual cingulum. The P3 differs from that of S. brachyurus in narrowing anteriorly rather than broadening, and in its more elongate buccal cingulum. It further differs from that of P. otibandus, W. bicolor, S. brachyurus, B. bandharr, D. lumholtzi, P. xanthopus, T. billardierii and H. puckridgi in being more elongate and in having more cuspules. It further differs from that of P. otibandus and W. bicolor in having a less anteriorly extensive lingual cingulum. It further differs from that of D. lumholtzi in having a proportionally narrower anterior cuspule and a less raised, narrower buccal cingulum, and in lacking an indentation in the main crest immediately posterior to the anterior cuspule and a posterobuccal cuspule. The P3 is additionally differentiated from that of W. bicolor in being longer relative to the length of M1. The P3 of D. buloloensis is distinguished from that of Wanburoo hilarus, Wabularoo prideauxi, Cookeroo hortusensis and Ganguroo robustiter in having fewer cuspules, and from W. hilarus, W. prideauxi and C. hortusensis in having less raised ridgelets and a lingual cingulum. It further differs from G. robustiter in having a narrower lingual cingulum and a proportionally narrower anterior cuspule, and from C. hortusensis in having a more buccally situated main crest.
The upper molars of D. buloloensis are most similar to those of the species of Dorcopsis and Dorcopsulus, D. fossilis, W. novaeguineae and W. hilarus. They differ from those of the species of D. fossilis, Dorcopsis and Dorcopsulus in being larger, and from D. fossilis in having slightly narrower interloph valleys in M1 and M2. The upper molars of D. buloloensis are slightly larger than those of W. novaeguineae, and have a slightly higher postparacrista and premetacrista. The upper molars additionally differ from those of D. vanheurni and W. novaeguineae in having a more prominent precingulum. The M1 and M2 of D. buloloensis differ from those of W. bicolor, S. brachyurus, P. xanthopus, D. lumholtzi and T. billardierii in having more prominent stylar cusps C and D. The upper molars are differentiated from W. bicolor, P. xanthopus and T. billardierii in being lower crowned, broader relative to length, and more rounded in occlusal view. They further differ from W. bicolor in having lower cristae, and from P. xanthopus and T. billardierii in having a lower postprotocrista, and a higher postparacrista and premetacrista that deflect slightly lingually into the interloph valley rather than being restricted to the buccal edge of the loph. The upper molars differ from those of S. brachyurus in having a more anteriorly prominent precingulum, particularly where it meets the preparacrista. The upper molars differ from those of D. lumholtzi, Bohra planei, B. bila and B. bandharr in being larger and in having slightly more raised postprotocrista, postparacrista, postmetacrista and postmetaconulecrista and a deeper posterior basin, and additionally from B. bandharr in having more lingually curved postparacrista and premetacrista. The M2 and M3 further differ from those of D. lumholtzi in having a broader metaloph relative to the width of the protoloph. The upper molars differ from those of P. otibandus in being smaller, with a premetacrista present. The M1 of D. buloloensis differs from that of W. hilarus, W. prideauxi, C. hortusensis and G. robustiter in having the stylar cusp C present as a narrow crest rather than a distinct cusp. The upper molars differ from both and W. prideauxi and G. robustiter in being more rounded, and from those of G. robustiter in having less anteriorly convex loph crests, though this may be related to different stages of wear. The upper molars differ from those of C. hortusensis in having a more lophodont protoloph, wherein the preprotocrista is less distinct and merges into the anterior surface of the protoloph rather than extending into the protocrista. They are differentiated from those of H. puckridgi in being smaller, with a more lingually deflected postparacrista and a premetacrista present. The M4 of D. buloloensis is differentiated from that of W. prideauxi and C. hortusensis by being larger relative to the other molars, with a broader metaloph relative to the protoloph.
Lower dentition
The i1 of Dorcopsoides buloloensis is known from two specimens, the holotype (CPC6774) and NHMD 193282, in which it is moderately worn and heavily worn, respectively (, ). The i1 is procumbent, orientated moderately anterodorsally relative to the angle of the proximal section of the diastema. It is small, lanceolate and transversely compressed, about twice as tall as it is wide at the base of the crown. In buccal (lateral) view the enamel-covered portion of i1 is similar in length to p3 and is approximately triangular, with a gently convex dorsal margin. The gently, smoothly convex buccal surface is completely covered by a layer of enamel. The buccal enamel layer wraps around the ventral edge and onto the ventral one-tenth of the lingual surface, forming a low, thick ventral crest. The remaining nine-tenths of the lingual surface are enamel-free.
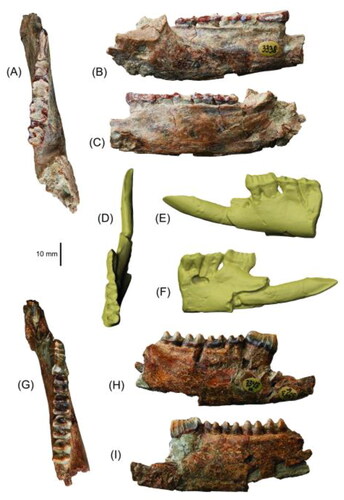
Fig. 4. Partial lower dentaries in A, D, G, occlusal; B, E, H, buccal; and C, F, I, lingual views. A–C, Partial right dentary of D. buloloensis NHMD 193278 with p3–m4; D–F, scan image of cast of partial left dentary of D. buloloensis holotype CPC 6774 (cast registered as AM F2297) with i1 and p3–m2; and G–I, partial right dentary of Watutia novaeguineae NHMD 193266 with p3–m4.
The p3 of D. buloloensis is known from three specimens: two slightly worn (CPC6774 and NHMD 193280) (, ) and one heavily worn (NHMD 193278) (). It is narrow, elongate, and blade-like, with a tall main crest linked by five cuspules. Its broadest point is across the anterior cuspule, gently narrowing to a distinct waist immediately anterior to the posterior-most cuspule, then abruptly broadening around the swollen base of the posterior-most cuspule. In occlusal view the main crest is straight between the anterior-most cuspule and the fourth cuspule, then curves gently lingually to the posterior cuspule. The main crest extends anteriorly through the anterior cuspule and down the anterior surface to the base of the crown, and similarly through the posterior cuspule and down the posterolingual edge. The anterior and posterior cuspules are subequal in height, with the second to fourth cuspules slightly lower. Narrow, raised buccal and lingual ridgelets extend ventrally from all but the posterior cuspule; the ridgelet from the anterior cuspule is most raised and distinct, while that of the fourth cuspule is very fine and slight. The ridgelets from the anterior cuspule slant posteriorly before extending straight ventrally.
The lower molars of D. buloloensis are known from four specimens: the holotype, which is a dentary preserving heavily worn m1 and m2 (CPC 6774) (); a fractured and moderately worn m2 and m3 and intact, slightly worn m4 (NHMD 193280); a heavily worn m1, moderately worn m2 and m3, and partial m4 (NHMD 193282) (); and heavily worn m1–m4 (NHMD 193278) (). The lower molars are low crowned, oblong and fairly elongate. Absolute length and lophid width increase to m3 then decrease in m4. Trigonid and talonid widths are subequal in m1 and m2; the trigonid is slightly wider than the talonid in m3 and considerably wider in m4. The interlophid valley is slightly narrower than the lophids in m1–m3 and considerably narrower in m4. Both lophid crests are gently posteriorly convex in occlusal view. The precingulid is small and very slightly anterobuccally convex. The distinct, raised paracristid curves anterobuccally from the base of the premetacristid, meets the precingulid slightly buccal to the midline of the tooth, and extends posteriorly then posterobuccally to the protoconid. The thick, low premetacristid extends straight from the anterolingual margin of the protolophid to the metaconid. The cristid obliqua originates mesially on the posterior surface of the protolophid, drops into the interlophid valley, then extends posterobuccally with a slight curve to merge into the hypolophid just beneath the hypoconid. The preentocristid is very thick, low and indistinct. The postcingulid is present just above the base of the crown on m2 as a slight swelling, on m3 as a broader swelling with a very slight shelf, and on m4 as a very thin lip across the posterior base.
The i1 of D. buloloensis differs from that of all compared species except Hadronomas puckridgi in being less elongate, though this is probably the result of wear. It is most similar to that of Setonix brachyurus, Dorcopsoides fossilis, the species of Dorcopsis and Dorcopsulus, and H. puckridgi. It is differentiated from that of the species of Dorcopsis and Dorcopsulus and from Walabia bicolor, Dendrolagus lumholtzi, Petrogale xanthopus and Thylogale billardierii in having a lower, thicker ventrolingual crest. It further differs from W. bicolor and T. billardierii in being angled more dorsally. The i1 differs from that of Wanburoo hilarus in being less tall, and from that of Gangaroo robustiter in being taller and more transversely compressed.
The p3 of D. buloloensis is most similar to that of D. fossilis and the species of Dorcopsis and Dorcopsulus. It differs from D. fossilis in having slightly less raised ridgelets and a slightly more distinct posterior waist, and from the species of Dorcopsis and Dorcopsulus in lacking a gently anterolingually curved main crest. It further differs from the species of Dorcopsis in being relatively shorter and having fewer cuspules, and from the species of Dorcopsulus in having a more distinct waist immediately anterior to the posterior cuspule. It additionally differs from that of Dorcopsoides macleayi in having much smaller indentations in the main crest between each cuspule. The p3 is distinguished from that of Watutia novaeguineae () by having a larger, more anteriorly prominent anterior cuspule and less raised ridgelets from the second, third and fourth cuspules. The p3 differs from that of all compared taxa except the species of Dorcopsis, Watutia and Dorcopsoides in having a distinct posterior waist. It further differs from that of Protemnodon otibandus, W. bicolor, Bohra bila, P. xanthopus, T. billardierii, S. brachyurus, Nombe nombe, D. fossilis, W. novaeguineae, H. puckridgi, Wabularoo prideauxi, Cookaroo hortusensis and G. robustiter in being relatively narrower and more elongate, and from P. otibandus, W. bicolor, B. bila, P. xanthopus, T. billardierii, S. brachyurus and N. nombe in having more cuspules and a narrower posterior cuspule relative to the anterior cuspule. It additionally differs from W. bicolor, B. bila, P. xanthopus and T. billardierii in having more raised ridgelets. It is distinct from that of D. lumholtzi in having a relatively narrower anterior cuspule and lacking an indentation in the main crest immediately posterior to the anterior cuspule. The p3 further differs from that of H. puckridgi in lacking a slight buccal cingulid, from W. hilarus, W. prideauxi, C. hortusensis and G. robustiter in having a larger, more anteriorly prominent anterior cuspule, and from W. hilarus and W. prideauxi in having less raised ridgelets. It additionally differs from W. prideauxi and C. hortusensis in having fewer cuspules, and from W. hilarus in lacking a posterolingually curved and projected posterior.
The lower molars of D. buloloensis are most similar to those of D. fossilis, W. novaeguineae and the species of Dorcopsis. They differ from those of D. fossilis in being larger. They differ from those of W. novaeguineae () in having a lower, less distinct premetacristid and a postcingulid, from species of Dorcopsis and Dorcopsulus in being larger, and from the species of Dorcopsulus in having a more anteriorly prominent paracristid, a less buccally situated cristid obliqua, and a postcingulid. The lower molars of D. buloloensis differ from those of N. nombe in having a higher, more distinct paracristid and cristid obliqua, particularly the paracristid, which is raised into a distinct, curved crest that extends through the paraconid into the protolophid (rather than merging into the anterior of the protolophid just below the paraconid). They further differ from those of N. nombe in having a gently posteriorly convex protolophid and in lacking a slight postprotocristid. The lower molars are differentiated from those of P. otibandus, W. bicolor, P. xanthopus and T. billardierii in being lower crowned, and from W. bicolor, Bohra bandharr, B. bila, D. lumholtzi, P. xanthopus, T. billardierii and S. brachyurus in being larger. They differ from those of P. otibandus and H. puckridgi in their smaller size. The lower m2–m4 differ from those of W. bicolor, P. xanthopus, T. billardierii, S. brachyurus, H. puckridgi, W. hilarus, W. prideauxi, C. hortusensis and G. robustiter in having a postcingulid. The m1 and m2 of D. buloloensis differ from that of P. otibandus, W. bicolor, W. hilarus, W. prideauxi and G. robustiter in being proportionally narrower. The lower molars are additionally differentiated from those of W. bicolor, D. lumholtzi, P. xanthopus, H. puckridgi and W. hilarus in having a less buccally situated cristid obliqua, and from W. bicolor in having a lower, less distinct preentocristid. They further differ from those of B. bandharr, D. lumholtzi and N. nombe in having a lower, less distinct premetacristid. The lower molars additionally differ from those of W. hilarus, W. prideauxi, C. hortusensis and G. robustiter in having a more anterolingually prominent paracristid.
Dentary
The dentary of Dorcopsoides buloloensis is known from two partial specimens: a cast of the holotype, which is a left dentary preserving the posterolateral quarter of the section of dentary below the diastema and the mandibular corpus anterior to m3 (CPC 6774) (); and a partial right dentary preserved from immediately anterior to the anterior mental foramen to the anteroventral margins of the masseteric and pterygoid fossae (NHMD 193278) (). The diastema is anteroposteriorly short, being subequal to the combined length of the p3 and m1. The posterior of the diastema is level, while the anterior is not known. The ventral margin of the dentary beneath the diastema is not known. The anterior mental foramen is situated on the buccal/lateral surface of the anterior dentary, immediately anteroventral to the anterior root of the p3. The mandibular corpus is deep and narrow, and is slightly deeper beneath the m4 than beneath the p3 such that the tooth row is very slightly anteroventrally inclined. Its ventral margin is level anteriorly and curves gently dorsally beneath the ascending ramus. A small posterior mental foramen is present beneath the m2 on the buccal surface. The buccinator sulcus is fairly narrow and shallow and extends with a moderate posteroventral tilt from the buccal surface by the anterior root of the p3 to beneath the m2/m3 margin. The posterior margin of the tooth row is slightly anterior to the anteroventral base of the ascending ramus. The anterior of the area of insertion of the intermediate (middle) masseter, along the anteroventral margin of the masseteric fossa, is deep, gently dorsobuccally concave, and demarcated from the posterior portion of the mandibular corpus by a low ridge.
The dentary of D. buloloensis is intermediate in size between that of Dorcopsoides fossilis and Nombe nombe. It differs from that of all compared taxa except N. nombe, the species of Dorcopsulus, Wanburoo hilarus, Wabularoo prideauxi, Cookeroo hortusensis and Ganguroo robustiter in having a distinct posterior mental foramen. It further differs from that of Dorcopsis luctuosa in having a slightly more posterodorsally situated anterior mental foramen, and from D. fossilis in having a shorter diastema and a more posterodorsally situated anterior mental foramen. It has a shallower mandibular corpus than that of Watutia novaeguineae. The dentary of D. buloloensis differs from that of Protemnodon otibandus, Walabia bicolor, Petrogale xanthopus and Thylogale billardierii in having a shorter diastema and a slightly anteroventrally inclined tooth row, and from W. bicolor, P. xanthopus, T. billardierii, Hadronomas puckridgi and C. hortusensis in having a shallower mandibular corpus, and from W. bicolor, P. xanthopus and T. billardierii in having an intermediate masseter insertion with a more distinct anteroventral ridge. It is differentiated further from that of W. bicolor, P. xanthopus and Setonix brachyurus by its less anteroventrally inclined diastema, and from P. otibandus, W. bicolor, P. xanthopus, T. billardierii and G. robustiter in having a more posteriorly situated anterior mental foramen. The dentary differs from that of Bohra bandharr in having a deeper mandibular corpus, from Bohra bila in having a more posterodorsally situated anterior mental foramen, from Dendrolagus lumholtzi and B. bila in having a less convex ventral margin, and from D. lumholtzi in having a more anterodorsally situated anterior mental foramen, and an intermediate masseter insertion with a more distinct anteroventral ridge. The dentary is further differentiated from that of W. prideauxi and G. robustiter in having a less convex ventral margin.
Dorcopsini gen. indet.
1967, Protemnodon buloloensis, Plane pp. 48–50, 63.
2023, Protemnodon buloloensis, Prideaux & Warburton, p. 65.
Referred material
UCMP 45345, a partial adult skeleton; root of I1; thoracic vertebrae T8–12 and lumbar vertebra L1; parts of 11 ribs; right calcaneus with calcaneal tuberosity proximal section intact; lateral side of talus; ectocuneiform; most of distal half of metatarsal II; metatarsal Ill; proximal and distal ends of metatarsal IV; metatarsal IV; metatarsal V; proximal plantar sesamoid for metatarsals IV and V; sesamoids for proximal fourth phalanx; distal fourth phalanx; proximal and middle fifth phalanges. UCMP 70132, a right maxilla fragment preserving M3.
Referred material locality, unit and age
UCMP 45345, Woodard 2 (UCMP V5573) and UCMP 70132, Awe Fauna Type Locality (UCMP V6234), Papua New Guinea; Otibanda Formation, late Pliocene, 2.9 ± 0.4 Ma (Hoch & Holm Citation1986).
Remarks
UCMP 45345 was described and allocated to ‘Protemnodon’ buloloensis by Plane (Citation1967). Plane justified the allocation on the basis that the material was significantly smaller than that of Protemnodon otibandus and so most likely belonged to ‘P.’ buloloensis, which was then the only other macropodid known from the Otibanda Formation. However, Watutia novaeguineae, which is similarly sized to ‘P.’ buloloensis, was described by Flannery et al. (Citation1989). As UCMP 45345 consists of postcranial material and a non-diagnostic partial i1, it could belong to either Dorcopsoides buloloensis or W. novaeguineae. The specimen is substantially larger than Bohra planei, the only non-dorcopsin macropodine from Otibanda. As such, we allocate it to Dorcopsini gen. indet. Should associated craniodental and postcranial material of either species be excavated from the Otibanda Formation, this allocation will be revisited.
UCMP 70132 was collected by Michael D. Plane in 1963 (MP61, Acc 2201) from a ‘blue to grey claystone on a cliff about 20 feet above the western bank of the Watut River’ (Plane Citation1967, p. 63). The specimen was discussed by Prideaux & Warburton (Citation2023) and considered to probably represent a large dorcopsin on the basis of size, absence of a distinct stylar cusp C and a precingulum with an abruptly terminating lingual margin. We concur with this assessment and in the absence of features to confidently allocate the specimen to either W. novaeguineae or D. buloloensis we allocate it here to Dorcopsini gen. indet.
Results
Phylogenetic analysis
Parsimony analysis of the 65 species and 120 characters (47 dental, 73 osteological) examined 1,388,638,477 different tree arrangements, returning 1020 most parsimonious trees with a length of 585 steps. In the strict consensus tree () various nodes are collapsed. Within Macropodidae, the two species of Gumardee place sister to all other macropodids with strong support from bootstrapping/jackknifing (81/88). A five-way polytomy consisting of Wabularoo prideauxi, Wabularoo naughtoni, Wanburoo hilarus, subfamily Sthenurinae—a clade of Hadronomas puckridgi + Rhizosthenurus flanneryi + Archaeosimos cegsai + Metasthenurus newtonae + Sthenurus andersoni—and a clade of subfamily Macropodinae, receives low–moderate support (58/64). This polytomy sits sister to subfamily Lagostrophinae (Tjukuru wellsi + Lagostrophus fasciatus + Troposodon minor) with strong node support (91/96). Within subfamily Macropodinae, tribe Dorcopsini (Dorcopsoides fossilis + Dorcopsoides buloloensis + Watutia novaeguineae + the species of Dorcopsis and Dorcopsulus) receives very good support (91/90) as sister to all other macropodines. Tribe Macropodini sits in a six-way polytomy with Setonix brachyurus, Thylogale thetis (Lesson & Garnot, Citation1827), T. billardierii, the species of Petrogale Gray, Citation1837, and a clade of Nombe nombe + subtribe Dendrolagina (the species of Bohra and Dendrolagus). Nombe nombe sits basal to Dendrolagina with no support from bootstrapping nor jackknifing.
Fig. 5. Strict consensus of 220 most parsimonious trees showing the evolutionary relationships of members of the tribe Dorcopsini within Macropodidae. Subfamily Macropodinae and the three contained tribes are indicated by brackets on the right. Where node support from bootstrapping and jackknifing is greater than 50, this is indicated by the numbers on the branch to the left of the node (bootstrapping/jackknifing).
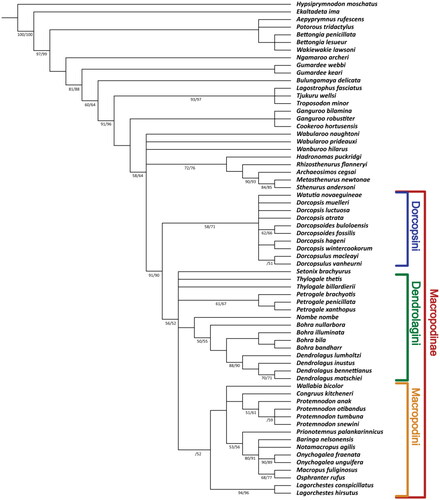
The relationships within Dorcopsini are poorly resolved in the strict-consensus tree (). Within the tribe a seven-part polytomy of W. novaeguineae, Dorcopsis muelleri, Dorcopsis luctuosa, Dorcopsis atrata, Dorcopsis hageni + Dorcopsis wintercookorum, Dorcopsoides fossilis + Dorcopsoides buloloensis, and Dorcopsulus vanheurni + Dorcopsulus macleayi receives moderate node support (58/71). The genus Dorcopsis is not returned monophyletically. The pairing of D. fossilis + D. buloloensis is moderately supported (62/66). The pairing of D. vanheurni and D. macleayi has only low jackknifing support (51).
In this analysis the species of tribe Dorcopsini are united by one unique synapomorphy: marked buccal constriction of P3 immediately anterior to the posterolingual cusp (char. 44). Dorcopsins are also linked by five homoplastic synapomorphies: a small postglenoid process (char. 6); partially or fully confluent masseteric and dental canals (char. 29); M1 with the stylar cusp C reduced to a stylar crest (char. 51); a procumbent, thin and elongate i1 (char. 60); and marked medial projection of the sustentaculum tali (char. 90). A small postglenoid process is also present in this analysis in the propleopine Ekaltadeta ima, the potoroid Bettongia penicillata, three species of basal macropodid Ganguroo Cooke, Citation1997, and both included species of macropodin Onychogalea Gray, Citation1841. Partially or fully confluent masseteric and dental canals are also scored for all non-macropodids in the analysis. M1 with a stylar cusp C reduced to a crest was present in W. hilarus, H. puckridgi, both included species of Thylogale, Petrogale brachyotis (Gould, Citation1841b) and Petrogale penicillata (Gray, Citation1827) and Bohra illuminata Prideaux & Warburton, Citation2008, Bohra bandharr and Bohra bila. A procumbent, thin and elongate i1 is present for all non-macropodids, lagostrophines and basal macropodids in the analysis, as well as the included species of Lagorchestes Gould, Citation1841a and Onychogalea. A marked medial projection of the sustentaculum tali is present in the species of Bohra and Dendrolagus except Dendrolagus matschei Förster & Rothschild, Citation1907, the species of Protemnodon, and Onychogalea fraenata (Gould, Citation1841b).
The analysis pairs D. fossilis and D. buloloensis with two homoplastic synapomorphies: a medium-length premolar (P3/p3 divided by M1/m1 length = 1.3–1.599) (char. 63); and a weak or absent premetacristid (char. 70). In this analysis a medium-length premolar is also present in non-macropodine taxa in Hypsiprymnodon moschatus, Potorous tridactylus (Kerr, Citation1792), Bulungamaya delicata, Ganguroo bilamina, Cookeroo hortusensis, W. hilarus, both included species of Ganguroo, and the three included crown sthenurines. Within Macropodinae, this state is present in N. nombe, B. illuminata, B. bandharr, B. bila, Dendrolagus lumholtzi, Setonix brachyurus, and the four included species of Protemnodon. A weak or absent premetacristid is shared in this analysis with H. moschatus, T. wellsi, the three crown sthenurines, both included species of Thylogale, Petrogale brachyotis and P. xanthopus, Dendrolagus bennettianus and D. matschei, S. brachyurus, and all crown macropodins except Protemnodon otibandus, Protemnodon tumbuna and Protemnodon snewini.
Nombe nombe and the species of Bohra and Dendrolagus are united by a single homoplastic synapomorphy: a masseteric canal extending anteriorly to below the posterior cheek teeth (char. 30). This state is shared with the three lagostrophines, B. delicata, both included species of Ganguroo, W. prideauxi, W. hilarus, H. puckridgi and R. flanneryi. A notable novel character in this analysis is the state of the paracristid of m3 and m4 merging into the anterior surface of the protolophid at or near protoconid, rather than being raised into distinct crest that clearly extends into the protolophid crest (char. 79). This state is here recognized only in N. nombe, B. bandharr and B. bila.
Discussion
Taxonomy and relationships
We place the species formerly referred to as ‘Silvaroo’ buloloensis in the genus Dorcopsoides with the only other congener, Dorcopsoides fossilis. This is done on the basis of their close morphological similarity to the exclusion of other taxa and their placement as sister taxa in the phylogenetic analysis (). The pairing of the two species is only supported by two homoplastic synapomorphies, but this is unsurprising given the fragmentary nature of the fossils of Dorcopsoides buloloensis. The placement of D. buloloensis within Dorcopsini has very good node support and the taxonomic analysis convincingly demonstrates the close affinity with D. fossilis.
Because there are diagnostic features in both upper and lower dentitions and the dentary of D. buloloensis, we do not deem it probable that the two are conspecific. Moreover, they are separated temporally by several million years. The possibility that D. buloloensis would be better placed in a new genus was considered. However, due to the absence of prominent diagnostic features that could constitute a genus-level distinction, we consider this a less favourable option. Although the dentition and dentary of D. buloloensis are considerably larger than those of D. fossilis, they do not differ to a degree that should constitute a genus-level distinction in macropodines. We propose that tribe Dorcopsini be expanded to include an additional fossil taxon, D. buloloensis, and support the conclusions of Prideaux & Warburton (Citation2023) in placing Watutia novaeguineae in Dorcopsini. This does not alter the current morphological definition of the tribe as it was erected by Prideaux & Warburton (Citation2010).
The relationships within Dorcopsini are not so well resolved by this strict-consensus tree (), in which there are only four fossil dorcopsins, three known only from craniodental material. If their evolution is to be better understood, the publication of an inclusive molecular phylogenetic study of dorcopsins is essential. A study in the fashion of that of Eldridge et al. (Citation2018) on Dendrolagus or Potter et al. (Citation2012) on Petrogale is sorely lacking for such a key group in the evolution of modern kangaroos. The lack of clarity in dorcopsin phylogenetics also highlights the need for more complete material of known fossil dorcopsins and the discovery of new fossil dorcopsin taxa.
The association of Nombe nombe with the dendrolagins is intriguing but more complete material is needed before its phylogenetic relationships can be ascertained. The clade is supported by only a single homoplasious synapomorphy. Further, the relationship is not supported by bootstrapping nor jackknifing (). We consider it an unaffiliated basal macropodine.
Evolutionary implications
The taxonomic and phylogenetic hypotheses put forward here indicate a late Pliocene dorcopsin presence in New Guinea. However, it seems very unlikely that either Dorcopsoides buloloensis or Watutia novaeguineae are the ancestors of modern dorcopsins. Species of the genus Dorcopsis had already arisen by the early Pliocene, as demonstrated by Dorcopsis wintercookorum, the only known fossil species of Dorcopsis, which occurs in the well-dated early Pliocene Hamilton Local Fauna (4.46 ± 0.1 Ma) (Turnbull et al. Citation2003) in southwestern Victoria. It thus seems likely that Dorcopsis and Dorcopsulus diverged in the late Miocene at the earliest, with plesiomorphic species of both genera coeval with the late Miocene (6–10 Ma) D. fossilis. This is in keeping with the most recent molecular divergence estimate, which placed the divergence of Dorcopsis and Dorcopsulus in the late Miocene (Westerman et al. Citation2022). These findings instead show the occurrence of a more substantial late Neogene dorcopsin radiation than previously thought, in terms of taxonomic diversity and body mass.
It is highly likely that the radiation of dorcopsins was influenced by climatic conditions and associated changes to vegetation. It also appears that living dorcopsins provide an incomplete picture of past diversity and habitat associations, as is the case with dendrolagins (Prideaux & Warburton Citation2023). Following the aforementioned drying of the Australian climate in the mid–late Miocene, there was a period of increased humidity, rainfall and forest expansion in Australia during the early Pliocene, often called the ‘Warm Period’ or ‘Humid Interval’ (McGowran et al. Citation2004, Sniderman et al. Citation2016, Karatsolis et al. Citation2020). Prior to this, the only known pre-Pliocene dorcopsin, Dorcopsoides fossilis, is considered to have inhabited xeric scrubland and open woodland in late Miocene central Australia (Woodburne Citation1967, Murray Citation1994, Megirian et al. Citation1996). All dorcopsins from during or after the early Pliocene are associated with higher-rainfall, more vegetated habitats. Dorcopsoides buloloensis and W. novaeguineae are believed to have lived in tropical lowland savannah-woodland (Plane Citation1967, Flannery et al. Citation1989), while all species of Dorcopsis and Dorcopsulus inhabit or inhabited rainforest environments (Flannery Citation1990b, Flannery et al. Citation1992). To improve understanding of the palaeoecological development of dorcopsins to any level of certainty, however, the fossil record must be expanded from the currently known fragmentary remains from three geographically and temporally isolated sites. The early Pliocene Warm Period has been highlighted as a possible influence on the radiation of tree-kangaroos (Prideaux & Warburton Citation2023).
Supplemental Data 2
Download Text (26.4 KB)Supplemental Data 1
Download MS Word (28.1 KB)Acknowledgements
David Stemmer (SAMA), Matthew McCurry (Australian Museum) Natalie Schroeder (CPC), Tim Denham and Mary-Jane Mountain (ANU), Scott Hocknull and Heather Janetzki (QM), Jim Anamiato and Bulisa Iova (PNG), Pat Holroyd and Kevin Padian (UCMP), Ella Hoch and Bent Lindow (NHMD) and all their respective institutions are thanked for access to specimens. Jacob van Zoelen and OzBoneViz shared scans of rare dorcopsins. The documented fossils were collected from lands of the Bulolo district people in Papua New Guinea.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplemental data
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Ameghino, F., 1889. Actas de la Academia nacional de ciencias de la República Argentina en Cordoba. Contribucion al conocimiento de los mamiferos fosiles de la República Argentina: Obra escrita bajo los auspicios de la Academia nacional de ciencias de la República Argentina Para ser presentada á la Exposicion universal de Paris de 1889. 6. P.E. Coni é hijos, Buenos Aires, 1006 pp.
- Aplin, K.P., Baverstock, P.R. & Donnellan, S.C., 1993. Albumin immunological evidence for the time and mode of origin of the New Guinean terrestrial mammal fauna. Science in New Guinea 19, 131–145.
- Archer, M., 1979. Wabularoo naughtoni gen. et. sp. nov., an enigmatic kangaroo (Marsupialia) from the middle Tertiary Carl Creek Limestone of northwestern Queensland. Results of the Ray E. Lemley Expeditions, part 4. Memoirs of the Queensland Museum 19, 299–307.
- Archer, M. & Flannery, T.F., 1985. Revision of the extinct gigantic rat kangaroos (Potoroidae: Marsupialia), with description of a new Miocene genus and species and a new species of Propleopus. Journal of Palaeontology 59, 1331–1349.
- Beck, R.M., 2017. The biogeographical history of non-marine mammaliaforms in the Sahul region. In Handbook of Australasian Biogeography. Ebach, M.C., ed. CRC Press, Boca Raton, 329–366.
- Beck, R.M.D., Voss, R.S. & Jansa, S.A., 2022. Craniodental morphology and phylogeny of marsupials. Bulletin of the American Museum of Natural History 457, 1–352.
- Black, K.H., Archer, M., Hand, S.J. & Godthelp, H., 2012. The rise of Australian marsupials: a synopsis of biostratigraphic, phylogenetic, palaeoecologic and palaeobiogeographic understanding. In Earth and Life: global Biodiversity, Extinction Intervals and Biogeographic Perturbations through Time. Talent, J.A., ed. Springer, Dordrecht, 983–1078.
- Burk, A., Westerman, M. & Springer, M., 1998. The phylogenetic position of the musky rat-kangaroo and the evolution of bipedal hopping in kangaroos (Macropodidae: Diprotodontia). Systematic Biology 47, 457–474.
- Butler, K., Travouillon, K.J., Price, G.J., Archer, M. & Hand, S.J., 2016. Cookeroo, a new genus of fossil kangaroo (Marsupialia, Macropodidae) from the Oligo-Miocene of Riversleigh, northwestern Queensland, Australia. Journal of Vertebrate Palaeontology 36, e1083029.
- Cascini, M., Mitchell, K.J., Cooper, A. & Phillips, M.J., 2019. Reconstructing the evolution of giant extinct kangaroos: Comparing the utility of DNA, morphology, and total evidence. Systematic Biology 68, 520–537.
- Cloos, M., Sapiie, B., Van Ufford, A.Q., Weiland, R.J., Warren, P.Q. & Mcmahon, T.P., 2005. Collisional delamination in New Guinea: The geotectonics of subducting slab breakoff. Geological Society of America Special Papers 400, 1–51.
- Collett, R., 1884. On some apparently new marsupials from Queensland. Proceedings of the Zoological Society of London 52, 381–389.
- Cooke, B.N., 1997. New Miocene bulungamayine kangaroos (Marsupialia: Potoroidae) from Riversleigh, northwestern Queensland. Memoirs of the Queensland Museum 41, 281–294.
- Cooke, B.N., 1999. Wanburoo hilarus gen. et sp. nov., a lophodont bulungamayine kangaroo (Marsupialia: Macropodoidea: Bulungamayinae) from the Miocene deposits of Riversleigh, northwestern Queensland. Records of the Western Australian Museum Supplement 57, 239–253.
- Cooke, B.N., Travouillon, K.J., Archer, M. & Hand, S.J., 2015. Ganguroo robustiter, sp. nov. (Macropodoidea, Marsupialia), a middle to early late Miocene basal macropodid from Riversleigh World Heritage Area, Australia. Journal of Vertebrate Palaeontology 35, e956879.
- Couzens, A.M.C. & Prideaux, G.J., 2018. Rapid Pliocene adaptive radiation of modern kangaroos. Science 362, 72–75.
- D’albertis, L.M., 1874. Characters of a new species of kangaroo (Halmaturus luctuosus). Proceedings of the Zoological Society of London 1874, 110.
- Dawson, L., 2004. A new fossil genus of forest wallaby (Marsupialia, Macropodinae) and a review of Protemnodon from eastern Australia and New Guinea. Alcheringa 28, 275–290.
- Dawson, L., Muirhead, J. & Wroe, S., 1999. The Big Sink Local Fauna: A lower Pliocene mammalian fauna from the Wellington Caves complex, Wellington, New South Wales. Records of the Western Australian Museum Supplement No 57, 265–290.
- de Miklouho-Maclay, N., 1885. On two new species of Dorcopsis from the south coast of New Guinea. Proceedings of the Linnean Society of New South Wales. 10, 145–150.
- Desmarest, A., 1804. Tableau Méthodique des mammifères. Nouveau Dictionnaire d'Histoire Naturelle 24, 5–58.
- Desmarest, A.G., 1822. Mammalogie, ou, Description des espèces de mammifères. 2. Chez Mme Veuve Agasse, Paris, France, 530. pp.
- Eldridge, M.D., Potter, S., Helgen, K.M., Sinaga, M.H., Aplin, K.P., Flannery, T.F. & Johnson, R.N., 2018. Phylogenetic analysis of the tree-kangaroos (Dendrolagus) reveals multiple divergent lineages within New Guinea. Molecular Phylogenetics and Evolution 127, 589–599.
- Flannery, T.F., 1990a. Dating the great New Guinea-Australia vicariance event: New evidence for the age of Australia’s Tertiary mammal fauna. In The De Vis Symposium. Memoirs of the Queensland Museum, Brisbane, 323 pp.
- Flannery, T.F., 1990b. Mammals of New Guinea. Robert Brown and Associates, Brisbane, 568. pp.
- Flannery, T.F., Archer, M. & Plane, M.D., 1983. Middle Miocene kangaroos (Macropodoidea: Marsupialia) from three localities in northern Australia, with a description of two new subfamilies. Bureau of Mineral Resources Journal of Australian Geology and Geophysics 7, 287–302.
- Flannery, T.F., Hoch, E. & Aplin, K., 1989. Macropodines from the Pliocene Otibanda Formation, Papua New Guinea. Alcheringa 13, 145–152.
- Flannery, T.F., Rich, T.H., Turnbull, W.D. & Lundelius Jr, E.L., 1992. The Macropodoidea (Marsupialia) of the Early Pliocene Hamilton Local Fauna. Fieldiana, Victoria, Australia, 1–37.
- Flannery, T.F. & Szalay, F.S., 1982. Bohra paulae, a new giant fossil tree kangaroo (Marsupialia: Macropodidae) from New South Wales, Australia. Australian Mammalogy 5, 83–94.
- Flower, W.H., 1867. On the development and succession of teeth in the Marsupialia. Philosophical Transactions of the Royal Society 157, 631–641.
- Föerster, F. & Rothschild, L.W.R., 1907. Description of a new tree kangaroo. Novitates Zoologicae. 14, 506–506.
- Goloboff, P.A., Farris, J.S. & Nixon, K.C., 2008. TNT, a free program for phylogenetic analysis. Cladistics 24, 774–786.
- Gould, J., 1841a. A Monograph of the Macropodidae, or Family of Kangaroos. 1. John Gould, London, 184 pp.
- Gould, J., 1841b. On five new species of kangaroo. Proceedings of the Zoological Society of London, 92–94.
- Gray, J.E., 1821. On the Arrangement of Vertebrose Animals. London Medical Repository, London, 296–310.
- Gray, J.E., 1827. A synopsis of the species of the class Mammalia. In The Animal Kingdom Arranged in Conformity with Its Organisation by the Baron Cuvier, Member of the Institute of France Etc. with Additional Descriptions of All the Species Hitherto Named, and of Many Not before Noticed. Griffith, E., Pidgeon, E. & Smith, C.H., eds. William Clowes, Charing Cross, London, UK, 185–206.
- Gray, J.E., 1837. Description of some new or little known Mammalia, principally in the British Museum collection. Magazine of Natural History and Journal of Zoology, Botany, Mineralogy, Geology and Meteorology 1, 577–587.
- Gray, J.E., 1841. Contributions towards the geographical distribution of the Mammalia in Australia, with notes on some recently discovered species, in a letter addressed to the Author. In Journals of Two Expeditions of Discovery in North-west and Western Australia during the Years 1837, 38, and 39, under the Authority of Her Majesty’s Government. Describing many newly discovered, important, and fertile districts, with observations on the moral and physical condition of the aboriginal inhabitants. Grey, G., ed. T. & W. Boone, London, 397–414.
- Gray, J.E., 1855. Description of a new species of Petrogale. Proceedings of the Zoological Society of London 22, 1854, 249–249.
- Grigg, G.C., Jarman, P.J. & Hume, I.D., 1989. Introduction. In Kangaroos, Wallabies and Rat-kangaroos. Grigg, G.C., Jarman, P.J. & Hume, I.D. eds., Surrey Beatty & Sons, Sydney, xiii–xiv.
- Groves, C.P. & Flannery, T.F., 1989. Revision of the genus Dorcopsis (Macropodidae: Marsupialia). In Kangaroos, Wallabies and Rat-Kangaroos. Grigg, G.C., Jarman, P.J. & Hume, I.D., eds. Surrey Beatty, Sydney, 117–128.
- Helgen, K., 2007. A taxonomic and geographic overview of the mammals of Papua. In The Ecology of Indonesia Series. The Ecology of Papua, Part One. Marshall, A.J. & Beehler, B.M., eds. Periplus Editions, Singapore, 689–749.
- Helgen, K.M., Wells, R.T., Kear, B.P., Gerdtz, W.R. & Flannery, T.F., 2006. Ecological and evolutionary significance of sizes of giant extinct kangaroos. Australian Journal of Zoology 54, 293–303.
- Heller, K.M., 1897. Zwei neue Beutelthiere aus Deutsch Neu Guinea nebst einer Aufzählung der bekannten papuanischen Säugethiere. Abhandlungen und Berichte der Königlichen Zoologischen und Anthropologisch-Ethnologischen Museen Dresden 66, 1–7.
- Hoch, E. & Holm, P.M., 1986. New K/Ar age determinations of the Awe Fauna Gangue, Papua New Guinea: Consequences for Papuaustralian Late Cenozoic biostratigraphy. Modern Geology 10, 181–195.
- Jackson, S.M. & Groves, C., 2015. Taxonomy of Australian Mammals. CSIRO Publishing, Clayton South, 529 pp.
- Janis, C.M., Buttrill, K. & Figueirido, B., 2014. Locomotion in extinct giant kangaroos: Were sthenurines hop-less monsters? PLoS One 9, e109888.
- Janis, C.M., Damuth, J., Travouillon, K.J., Figueirido, B., Hand, S.J. & Archer, M., 2016. Palaeoecology of Oligo-Miocene macropodoids determined from craniodental and calcaneal data. Memoirs of Museum Victoria 74, 209–232.
- Janis, C.M., O’Driscoll, A.M. & Kear, B.P., 2023. Myth of the QANTAS leap: Perspectives on the evolution of kangaroo locomotion. Alcheringa 47, 671–685.
- Johnson, K.A., Burbidge, A.A. & Mckenzie, N.L., 1989. Australian Macropodoidea: Status, causes of decline and future research and management. In Kangaroos, Wallabies and Rat-kangaroos. Grigg, G.C., Jarman, P.J. & Hume, I.D. eds., Surrey Beatty & Sons, Sydney, 641–657.
- Karatsolis, B.T., de Vleeschouwer, D., Groeneveld, J., Christensen, B. & Henderiks, J., 2020. The late Miocene to early Pliocene “Humid Interval” on the NW Australian shelf: Disentangling climate forcing from regional basin evolution. Paleoceanography and Paleoclimatology 35, e2019PA003780.
- Kear, B.P., 2002. Phylogenetic implications of macropodid (Marsupialia: Macropodoidea) postcranial remains from Miocene deposits of Riversleigh, northwestern Queensland. Alcheringa 26, 299–318.
- Kear, B.P., Lee, M.S.Y., Gerdtz, W.R. & Flannery, T.F., 2008. Evolution of hind limb proportions in kangaroos (Marsupialia: Macropodoidea). In Mammalian Evolutionary Morphology: A Tribute to Frederick S. Szalay. Sargis, E.J. & Dagosto, M. eds. Springer Science, New York, 25–35.
- Kear, B.P. & Pledge, N.S., 2007. A new fossil kangaroo from the Oligocene-Miocene Etadunna Formation of Ngama Quarry, Lake Palankarinna, South Australia. Australian Journal of Zoology 55, 331–339.
- Kerr, I.A.R. & Prideaux, G.J., 2022. A new genus of kangaroo (Marsupialia, Macropodidae) from the late Pleistocene of Papua New Guinea. Transactions of the Royal Society of South Australia 146, 295–318.
- Kerr, R., 1792. Bradypus tridactylus. In The Animal Kingdom, or Zoological System, of the Celebrated Sir Charles Linnæus. containing a Complete Systematic Description, Arrangement, and Nomenclature, of All the Known Species and Varieties of the Mammalia, or Animals Which Give Suck to Their Young. Class I: Mammalia. Linné, C.v., Gmelin, J.F., Kerr, R. & Archer, J., eds. J. Murray and R. Faulder, London, 101.
- Lesson, R.P., 1827. "Kangurus veterum". InVoyage autour du monde: exécuté par ordre du Roi, sur la corvette de Sa Majesté, la Coquille, pendant les années 1822, 1823, 1824, et 1825. Duperrey, L.-I., ed. Arthus Bertrand, Paris, 164.
- Lesson, R.P. & Garnot, P., 1827. Voyage autour du Monde, Enterprise par Ordre du Gouvernement sur la Corvette La Coquille, Zoologie. In Voyage autour du Monde, execute per ordre du Roi, sur la Corvette La Coquille de sa Majeste, pendant les annies 1822, 1823, 1824 et 1825. Duperrey, L.-I., ed. Firmin Didot for Arthus Bertrand, Paris.
- Linnaeus, C., 1758. Classis I: Mammalia. Systema naturae per regna tria naturae: secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Laurentii Salvii, Stockholm, 14–77.
- Long, J.A., Archer, M., Flannery, T. & Hand, S., 2002. Prehistoric Mammals of Australia and New Guinea: One Hundred Million Years of Evolution. University of New South Wales Press, Kensington, 280 pp.
- Luckett, W.P., 1993. Ontogenetic staging of the mammalian dentition, and its value for assessment of homology and heterochrony. Journal of Mammalian Evolution 1, 269–282.
- Macqueen, P., Goldizen, A.W., Austin, J.J. & Seddon, J.M., 2011. Phylogeography of the pademelons (Marsupialia: Macropodidae: Thylogale) in New Guinea reflects both geological and climatic events during the Plio‐Pleistocene. Journal of Biogeography 38, 1732–1747.
- Macqueen, P., Seddon, J.M., Austin, J.J., Hamilton, S. & Goldizen, A.W., 2010. Phylogenetics of the pademelons (Macropodidae: Thylogale) and historical biogeography of the Australo-Papuan region. Molecular Phylogenetics and Evolution 57, 1134–1148.
- Marcus, L.F., 1962. A new species of Sthenurus (Marsupialia, Macropodidae) from the Pleistocene of New South Wales. Records of the Australian Museum 25, 299–304.
- Matschie, P., 1916. Die Verbreitung der Beuteltiere auf Neu Guinea mit einigen Bemerkungen über ihre Einteilung in Untergattungen. Mitteilungen aus dem Zoologischen Museum in Berlin 8, 257–308.
- Mcgowran, B., Holdgate, G.R., Li, Q.Y. & Gallagher, S.J., 2004. Cenozoic stratigraphic succession in southeastern Australia. Australian Journal of Earth Sciences 51, 459–496.
- Megirian, D., Murray, P.F. & Wells, R.T., 1996. The late Miocene Ongeva Local Fauna of central Australia. The Beagle: Records of the Museums and Art Galleries of the Northern Territory 13, 9–37.
- Müller, S., 1840. Bijdragen tot de Kennis van Nieuw-Guinea. In Verhandlingen over de natuurlijke Geschiedenis der Nederlandische Overzeesche Bezittingen, Door de Leden der Natuurkundige Commissie in Indie en Andere Schrijvers. Zoologie. Temminck, C.J., ed. S & J. Luchtmans and C. C. van Der Hoek, Leiden, 1–80.
- Murray, P.F., 1994. Palaeoecology of the Alcoota Local Fauna. CSIRO Rangelands Seminar, CSIRO, 1–17. Alice Springs.
- Owen, R., 1874. On the fossil mammals of Australia, Part VIII. Family Macropodidae: Genera Macropus, Osphranter, Phascolagus, Sthenurus and Protemnodon. Philosophical Transactions of the Royal Society 164, 245–288.
- Owen, R., 1877a. On a new species of Sthenurus, with remarks on the relation of the genus to Dorcopsis. Proceedings of the Zoological Society of London 1877, 352–361.
- Owen, R., 1877b. Researches on the Fossil Remains of the Extinct Mammals of Australia; with a Notice of the Extinct Marsupials of England. 1. J. Erxleben, London, 522. pp.
- Péron, F. & Lesueur, C.A., 1807. "Kangurus fasciatus". In Voyage de découvertes aux terres australes: exécuté par ordre de Sa Majesté l'empereur et roi, sur les corvettes le Géographe, le Naturaliste, et la goëlette le Casuarina, pendent les années 1800, 1801, 1802, 1803 et 1804. Freycinet, L.C.D.d., Lesueur, C.A., Petit, N.-M., Péron, F. & Baudin, N., eds. De L'Imprimerie Impériale, Paris, 114.
- Plane, M.D., 1967. Stratigraphy and vertebrate fauna of the Otibanda Formation, New Guinea. Bulletin of the Bureau of Mineral Resources, Geology and Geophysics, Australia 86, 1–64.
- Potter, S., Cooper, S.J.B., Metcalfe, C.J., Taggart, D.A. & Eldridge, M.D.B., 2012. Phylogenetic relationships of rock-wallabies, Petrogale (Marsupialia: Macropodidae) and their biogeographic history within Australia. Molecular Phylogenetics and Evolution 62, 640–652.
- Prideaux, G.J., 2000. Simosthenurus newtonae sp. nov., a widespread sthenurine kangaroo (Diprotodontia: Macropodidae) from the Pleistocene of southern and eastern Australia. Records of the South Australian Museum 33, 1–15.
- Prideaux, G.J., 2004. Systematics and evolution of the sthenurine kangaroos. University of California Publications in Geological Sciences 146, 1–623.
- Prideaux, G.J. & Tedford, R.H., 2012. Tjukuru wellsi, gen. et sp. nov., a lagostrophine kangaroo (Diprotodontia, Macropodidae) from the Pliocene (Tirarian) of northern South Australia. Journal of Vertebrate Palaeontology 32, 717–721.
- Prideaux, G.J. & Warburton, N.M., 2008. A new Pleistocene tree-kangaroo (Diprotodontia: Macropodidae) from the Nullarbor Plain of south-central Australia. Journal of Vertebrate Paleontology 28, 463–478. https://doi.org/10.1671/0272-4634(2008)28[463:ANPTDM]2.0.CO;2
- Prideaux, G.J. & Warburton, N., 2023. A review of the late Cenozoic genus Bohra (Diprotodontia: Macropodidae) and the evolution of tree-kangaroos. Zootaxa 5299, 1–95.
- Prideaux, G.J. & Warburton, N.M., 2010. An osteology-based appraisal of the phylogeny and evolution of kangaroos and wallabies (Macropodidae: Marsupialia). Zoological Journal of the Linnean Society 159, 954–987.
- Quoy, J.R.C. & Gaimard, P., 1824. Zoologie. In Voyage autour du monde, entrepris par ordre du roi. Exécuté sur les corvettes de S.M. l‘Uranie et la Physicienne, pendant les années 1817, 1818, 1819 et 1820. Freycinet, L.C.D.d., ed. Chez Pillet AîNé, Paris, 1–712.
- Quoy, J.R.C. & Gaimard, P., 1830a. "Kangurus brachyurus". In Voyage de la corvette l'Astrolabe exécuté par ordre du roi: pendant les années 1826–1827 & 1828–1829. Lesson, A., Quoy, J.R.C., Gaimard, P., de Boisduval, J.B.A.C. & Richard, A., eds. J. Tastu, Paris, 114–116.
- Quoy, J.R.C. & Gaimard, P., 1830b. Zoologie. In Voyage de Decouvertes de l'Astrolabe: exécuté par ordre du Roi, pendant les années 1826-1827-1828-1829. Dumont D’Urville, J.-S.b.-C.s., ed. Imprimerie Royale, Paris, 1–527.
- Ramsay, E.P., 1875. Description of a new genus and species of rat kangaroo, allied to the genus Hypsiprymnus, proposed to be called 'Hypsiprymnodon moschatus. Proceedings of the Linnean Society of New South Wales 1, 33–35.
- Sniderman, J.K., Woodhead, J.D., Hellstrom, J., Jordan, G.J., Drysdale, R.N., Tyler, J.J. & Porch, N., 2016. Pliocene reversal of late Neogene aridification. Proceedings of the National Academy of Sciences of the United States of America 113, 1999–2004.
- Thomas, O., 1922. New mammals from New Guinea and neighbouring islands. Annals and Magazine of Natural History 9, 261–265.
- Travouillon, K.J., Archer, M. & Hand, S.J., 2015. Revision of Wabularoo, an early macropodid kangaroo from mid-Cenozoic deposits of the Riversleigh World Heritage Area, Queensland, Australia. Alcheringa 39, 274–286.
- Travouillon, K.J., Butler, K., Archer, M. & Hand, S.J., 2022. Two new species of the genus Gumardee (Marsupialia, Macropodiformes) reveal the repeated evolution of bilophodonty in kangaroos. Alcheringa 46, 105–128.
- Turnbull, W.D., Lundelius Jr, E.L. & Archer, M., 2003. Chapter 18: dasyurids, perameloids, phalangeroids, and vombatoids from the Early Pliocene Hamilton Fauna, Victoria, Australia. Bulletin of the American Museum of Natural History 279, 513–540.
- Van Deusen, H.M., 1957. Results of the Archbold Expeditions. No. 76 a New Species of Wallaby (Genus Dorcopsis) from Goodenough Island, Papua. American Museum Novitates, 1–25.
- Van Ufford, A.Q. & Cloos, M., 2005. Cenozoic tectonics of New Guinea. AAPG Bulletin 89, 119–140.
- Walker, D., 1972. Bridge and Barrier: The Natural and Cultural History of Torres Strait. 3. Australian National University, Canberra, 437 pp.
- Westerman, M., Loke, S., Tan, M.H. & Kear, B.P., 2022. Mitogenome of the extinct Desert ‘rat-kangaroo’ times the adaptation to aridity in macropodoids. Scientific Reports 12, 5829.
- Woodburne, M.O., 1967. The Alcoota fauna, central Australia. An integrated palaeontological and geological study. Bulletin of the Bureau of Mineral Resources, Geology and Geophysics, Australia 87, 1–187.
- Woodburne, M.O., 1984. Wakiewakie lawsoni, a new genus and species of Potoroinae (Marsupialia: Macropodidae) of medial Miocene age, South Australia. Journal of Palaeontology 58, 1062–1073.

