ABSTRACT
Background
Intravenous glucocorticoid (IVGC) remains the main treatment for moderate-to-severe and active thyroid-associated ophthalmopathy (TAO). However, a substantial number (20–30%) of active moderate-to-severe TAO patients may not respond to IVGC. Some patients may have disease progression despite IVGC treatment or relapse after steroid withdrawal.
Objectives
To analyze risk factors for clinical activity and predictive factors for clinical outcomes of 4.5 g IVGC therapy in patients with moderate-to-severe TAO.
Design and methods
Our study was performed in two steps: step 1 involved 110 moderate-to-severe TAO patients and analyzed risk factors for TAO activity; step 2 involved 53 active moderate-to-severe TAO patients from step 1 who were treated with 4.5 g IVGC therapy and analyzed predictive factors for clinical outcomes of IVGC therapy. Multivariate logistic regression analysis was used to identify the independent predictors and establish the predictive model.
Results
Abnormal TRAb (OR = 4.717; P = 0.019) and the percentage of CD3+CD4+ T cell (OR = 1.092; P = 0.028) were independently associated with the activity of moderate-to-severe TAO patients. The pretreatment CAS-max in both eyes (OR = 7.221; P = 0.013) and the percentage of pretreatment CD3+T cell (OR = 0.718; P = 0.037) were independently associated with therapeutic efficacy. The pretreatment CAS-max in both eyes (OR = 156.53; P = 0.028) and the percentage of post-treatment CD3+T cell (OR = 0.554; P = 0.043) were independently associated with therapeutic efficacy. Besides, multivariable prediction models were established, which were better in the forecasting aspect than single-variable prediction models.
Conclusions
Based on the findings of this study, we should monitor the peripheral blood T cell subsets for TAO, which could be helpful to timely judge the condition of clinical manifestation and effect of treatment for TAO patients. Multivariable prediction models have been established, which have great significance for clinical work.
Introduction
Graves’ disease (GD) is a thyroid autoimmune disorder in which anti-thyrotropin (TSH) receptor autoantibodies (TRAb) with stimulating activity induce hyperthyroidism.Citation1 Graves’ ophthalmopathy (GO) also known as thyroid-associated ophthalmopathy (TAO),Citation2 is the main extrathyroidal manifestation of Graves’ disease.Citation3 TAO occurs in 25–50% of GD patients.Citation4,Citation5 When TAO patients develop ocular symptoms, approximately 80–90% of patients also have hyperthyroidism, and a few patients (approximately 10%) have hypothyroidism or euthyroidism.Citation4,Citation6 Its main clinical features are eyelid retraction, diplopia (caused by extraocular muscle dysfunction), protrusion, periorbital edema, conjunctival hyperemia, exposure keratitis, and compressive optic neuropathy.Citation7,Citation8 Patients with TAO suffer from impaired visual function, facial disfigurement, and at worst, irreversible visual loss caused by corneal ulceration or dysthyroid optic neuropathy, which result in a poor quality of life and socioeconomic status.Citation9,Citation10
The typical process of TAO is characterized by an active (inflammatory) phase, followed by a stabilization and remission (inactive) phase.Citation7,Citation11,Citation12 Although the etiology of TAO is not completely understood, lymphocytes and other mononuclear cells infiltrating the orbit are believed to drive an autoimmune response.Citation11,Citation13,Citation14 Previous studies have demonstrated that T cells played a dominant role in mediating the early inflammatory response, while the function of B cells was less emphasized.Citation15–17 Orbital fibroblasts (OFs) are considered to be the primary autoimmune target in TAO.Citation7,Citation18 The persistent inflammatory response in the orbit is postulated to be maintained by interactions between the OFs and infiltrated immunocompetent cells. Inflammatory cytokines produced by infiltrated cells activate OFs, which in turn produce chemokines and cytokines to mediate the recruitment, activation, and differentiation of immune cells.Citation7,Citation19 Several T cell subsets have been found in the orbit of TAO, of which CD4+T cells and CD8+T cells are the most common.Citation20,Citation21 Previous studies have shown that the total number of infiltrating cells in orbit correlates with the clinical activity score (CAS) of TAO.Citation22 Although the main immunopathogenic events are localized in the orbit, it is likely that the immunologic abnormalities (including T-cell abnormalities) in TAO patients extend beyond the orbit, and may thus be detectable in the peripheral blood.Citation23 In clinic, orbital tissue specimens are difficult to obtain, while peripheral blood specimens are relatively easy to get. We know little about T cell subsets in peripheral blood, and even less about the relationship between T cell subsets and TAO activity. This is something we need to explore.
Most TAO patients are diagnosed via ocular signs and thyroid antibodies, such as thyroid-stimulating hormone receptor antibody (TRAb). Greater than 70% of patients with GD are diagnosed with TAO via orbital magnetic resonance imaging (MRI).Citation4 Current available interventions for moderate-to-severe and active TAO consist of glucocorticoids (GCs), radiotherapy and some immune modulators, such as rituximab and teprotumumab.Citation24 Immunomodulators are too expensive to be widely used. So intravenous glucocorticoid (IVGC) remains the main treatment for moderate-to-severe and active GO. Clinically, IVGC treatment has acceptable outcomes for most patients in the active phase. Nevertheless, a substantial number (20–30%) of active moderate-to-severe TAO patients may not respond to GCs and adverse effects may occur after administration of high-dose or long-term GCs use. Some patients may have disease progression despite GCs treatment or relapse after steroid withdrawal.Citation25,Citation26 To date, the prognostic factors associated with treatment outcomes are not well understood. Therefore, it is important to identify the potential prognosticators of IVGC treatment. Moreover, the identification of modifiable predictors can provide practical intervention targets for the management of patients with TAO to improve treatment outcomes.
This present retrospective study aimed to analyze risk factors for activity and predictive factors for clinical outcomes of 4.5 g IVGC therapy in patients with moderate-to-severe TAO.
Subjects and Methods
Subjects
This was a retrospective study of patients with TAO admitted to the Shanghai Ninth People’s Hospital affiliated to Shanghai Jiaotong University School of Medicine from January 2016 to January 2022 of ChiCTR 2,000,028,935 study.
The inclusion criteria were as follows. The diagnosis was based on the Bartley diagnostic criteria.Citation27 Activity and severity assessments of TAO were based on the EUGOGO consensus statement.Citation28 Patients with a CAS greater than or equal to 3/7 were considered to have active TAO. Patients with moderate-to-severe TAO usually have any one or more of the following: lid retraction greater than or equal to 2 mm, moderate or severe soft tissue involvement, exophthalmos greater than or equal to 3 mm, and inconstant or constant diplopia.Citation28
The exclusion criteria were as follows: severe heart, liver, and renal insufficiency, such as myocardial ischemia or myocardial infarction, arrhythmia and cardiac insufficiency; alanine aminotransferase (ALT) and aspartate aminotransferase (AST) greater than or equal to 1.5 times the upper limit of the normal value; and estimated glomerular filtration rate less than 60 ml/min· (1.73 m2)−1. In addition, patients with other autoimmune diseases, tuberculosis, mental illness, and glaucoma; patients with a positive pregnancy test or who were breastfeeding; patients who received radioactive iodine treatment or a hepatitis vaccine within 3 months before enrollment; patients with ophthalmological signs caused by other diseases; patients who had received systemic immunotherapy for TAO within 1 month before enrollment, including oral or IVGC, or other immunosuppressants; patients who had orbital radiotherapy or orbital decompression; and patients with incomplete information were excluded.
This study was approved by the Ethics Committee of the Ninth People’s Hospital Affiliated to Shanghai Jiaotong University School of Medicine. The study protocol conformed with the ethical guidelines of the 1975 Declaration of Helsinki, as reflected by the a priori approval by the appropriate institutional review committee. Informed consent was obtained from all participants included in the study.
Data were collected from a total of 283 TAO patients which is a rare sample database in China. Our study was performed in two steps: step 1 involved 110 moderate-to-severe TAO patients and analyze risk factors for activity in patients with TAO; step 2 involved 53 active moderate-to-severe TAO patients from step 1 who were treated with 4.5 g IVGC therapy and analyze predictive factors for clinical outcomes of IVGC therapy.
Indications for IVGC therapy: a.) clinical activity score (CAS) ≥3/7; or b.) CAS < 3/7, but with increased signal intensity of extraocular muscles on contrast-enhanced orbital MRI.Citation29 Treatment protocols: A total of 4.5 g of intravenous methylprednisolone was administered (12-week therapy: 6 weekly infusions of 0.5 g, followed by 6 weekly infusions of 0.25 g; 4-week therapy: daily infusions of 0.5 g for 3 consecutive days per week for 2 weeks, followed by a daily infusion of 0.25 g for 3 consecutive days per week for 2 weeks).Citation30
Methods
Ophthalmic assessments, blood sample collection, and questionnaire completion were performed on the day before the first week of treatment and on the day after the last treatment.
A questionnaire about sociodemographic characteristics, medical history, family history, and lifestyle factors was administered during the interview. A single endocrinologist conducted the interviews and clinical examinations according to a standard protocol.
Blood samples were obtained between 6:00 a.m. and 9:00 a.m. after fasting for at least 8 hours. Blood was immediately refrigerated after phlebotomy, and after 2 hours, it was centrifuged, and the serum was aliquoted and frozen in a central laboratory. Triglycerides, total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C) levels were determined with an ADVIA2400 (Siemens). Serum total T3 (TT3), total T4 (TT4), free T3 (FT3), free T4 (FT4), thyroid stimulating hormone (TSH), and thyroid peroxidase antibodies (TPOAbs) were measured with a BECKMAN COULTER UniCel D×I800. Thyrotropin receptor antibody (TRAb), thyroglobulin antibody (TGAb), serum osteocalcin (OC), amino terminal propeptide of type I collagen (PINP), and carboxyterminal telopeptide of type I collagen (CTX) were measured with a Cobas 6000 (Roche). The detection of the percentage of the cluster of differentiation (CD) series in peripheral blood was conducted with a BD FACSCanto II flow cytometry system. The detection of immunoglobulins, such as IgG, was conducted by immunoturbidimetry.
The normal range of thyroid function: TSH: 0.56–5.91uIU/ml; FT3: 2.30–4.80 pg/ml; FT4: 0.62–1.24ng/dl; T3: 0.60–1.55 ng/ml; T4: 5.42–12.74ug/dl; TGAb: 0.00–115.00IU/ml; TPOAb: 0.00–9.00IU/ml; TRAb: 0.00–1.75IU/L. Ophthalmic assessments were jointly performed by an endocrinologist and an ophthalmologist. The CAS (spontaneous retrobulbar pain, pain on attempted up or down gaze, redness of the eyelids, redness of the conjunctiva, swelling of the eyelids, inflammation of the caruncle and/or plica, and conjunctival edema) was evaluated as previously reported.Citation31
Clinical Outcome Evaluation of Thyroid-Associated Ophthalmopathy
Clinical outcome of TAO was evaluated after treatment in respect to baseline conditions by the seven points CAS and was defined clinically improved when CAS improved by at least 2 points in at least one eye. Deterioration was defined by CAS worsening of at least 2 points or when dysthyroid optic neuropathy (DON) or corneal breakdown occurred. No changes were defined as the condition in which changes less than those described above occurred. For each assessment, patients who improved were considered as group I, patients who did not improve or who worsened were defined as group NI.Citation32
Statistical Analysis
Data analyses were performed using IBM SPSS Statistics, version 26 (IBM Corporation, Armonk, NY). A P value less than 0.05 indicated significance (2 sided). Continuous variables were summarized as the mean ± SD or median (interquartile range), and categorical variables were summarized as percentages (%). The Student’s t test or Mann – Whitney U test was used for continuous variables, and the Chi-square test was used for dichotomous variables. Binary Logistic regression analysis were performed to analyze risk factors for activity in patients with TAO and predictive factors for clinical outcomes of IVGC therapy, respectively. For analyzing combined effect of more than two variables on the dependent variable, multivariate logistic regression analysis was performed using backward stepwise procedures as variable selection method to minimize Akaike information criterion (AIC). Diagnostic accuracy was evaluated using receiver operating characteristic (ROC) curve analysis. The cutoff with the best compromise between sensitivity and specificity was assessed using Youden’s test.Citation33
Results
Step 1. The analysis of risk factors for activity in patients with TAO.
Baseline Characteristics of 110 Moderate-To-Severe TAO Patients
A total of 110 moderate-to-severe TAO patients (51 males, 59 females; mean age ± SD, 54.8 ± 11.9 years) were enrolled in the present study. The baseline characteristics were summarized in . Sixty (54.5%) patients had a history of smoking. Seventy-nine (71.8%) patients were treated with antithyroid medication. The mean duration of eye symptoms was 6 (3.75 ~ 12) months in the patients. The mean CAS-max of binoculus was 4(3 ~ 5). Thirty-one (28.2%) patients had a history of hypertension and twelve (10.9%) patients had a history of diabetes mellitus.
Table 1. Baseline characteristics of moderate-to-severe TAO patients.
Demographic and Clinical Characteristics in TAO Patients with Different Activity
Patients with a CAS greater than or equal to 3/7 were considered to have active TAO. According to this, all the patients were divided into two groups, including 23 non-active and 87 active TAO patients. As shown in , the mean age, the prevalence of hypertension, the percentage of patients with abnormal TRAb and the mean CAS (right eye, left eye, the maximum of binoculus) were higher in the active group than in the non-active group with a statistical difference (all P < 0.05). The levels of FT4, TT4, TPOAb and the percentage of patients with abnormal TPOAb were lower in the active group than in the non-active group with a statistical difference (all P < 0.05). The percentage of patients with abnormal FT3, TT3 were lower and the percentage of CD3+CD4+ T cell was higher in the active group than in the non-active group, but these measures were not statistically significant between the two groups (all 0.05<P < 0.1). No significant differences were found in terms of gender, the percentage of smokers, patients taking antithyroid medication, the duration of eye symptoms, the prevalence of diabetes mellitus, the levels of TSH, FT3, TT3, TGAb, TRAb, CD4+/CD8+ T cell, IgG, IgM, IgA, IgE, TC, TG, LDL, HDL, OC, PINP, β-CTX, the percentage of patients with abnormal TSH, FT4, TT4, TGAb, the percentage of CD3+T cell, CD3+CD8+T cell, CD19+ B cell, CD16+CD56+ NK cell (all P > 0.1) between the two groups.
Table 2. Demographic and clinical characteristics in moderate-to-severe TAO patients with different activity.
Predictive Factors for the Activity of TAO Patients
Multivariate logistic regression analysis was performed to identify the predictive factors for the activity of moderate-to-severe TAO patients (). The odds ratio (OR) of abnormal TRAb was 4.717 (95% confidence interval [CI], 1.287 ~ 17.284; P = 0.019) and that of CD3+CD4+ T cell was 1.092 (95% CI, 1.009 ~ 1.181; P = 0.028). These results suggested that abnormal TRAb and CD3+CD4+ T cell were independent risk factors for the activity of moderate-to-severe TAO patients.
Table 3. Predictive factors for activity of moderate-to-severe TAO patients by binary logistic regression analysis.
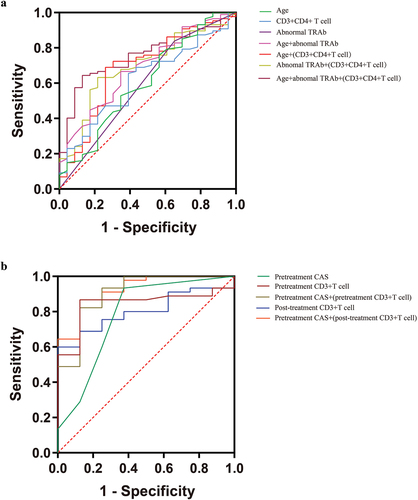
The ROC curve analysis of abnormal TRAb showed an area under curve (AUC) of 0.593 (95% CI, 0.456 ~ 0.731, P = 0.169). The ROC curve analysis of CD3+CD4+ T cell showed an AUC of 0.618 (95% CI, 0.497 ~ 0.739, P = 0.082). Furthermore, multivariable prediction models were established. The ROC curve analysis, with variables of age, abnormal TRAb and CD3+CD4+ T cell, showed an AUC of 0.756(95% CI, 0.658–0.853, p = 0.000), which was better than other prediction models ().
Step 2. The analysis of predictive factors for clinical outcomes of IVGC therapy.
According to the above principles of Clinical Outcome Evaluation of Thyroid-Associated Ophthalmopathy, all the patients were divided into two groups, including Group NI (n = 8) and Group I (n = 45).
The Analysis of Predictive Factors at Baseline for Clinical Outcomes of IVGC Therapy
Baseline Clinical Characteristics of Subjects According to Response After IVGC Therapy
As shown in , pretreatment CAS-max in both eyes and the percentage of CD19+ B cell were higher in the Group I than in the Group NI (both P < 0.05). The percentage of CD3+T cell was lower in the Group I than in the Group NI (P < 0.05). Other indicators were not statistically significant between the two groups (all P > 0.05).
Table 4. Baseline clinical characteristics of subjects according to response after IVGC therapy.
Predictive Factors for Response After IVGC Therapy by Binary Logistic Regression Analysis
Factors associated with the efficacy of IVGC therapy were first assessed by univariate analysis. As shown in , the percentage of pretreatment CD3+T cell (P = 0.011) and pretreatment CAS-max in both eyes (P = 0.019) were associated with the efficacy of IVGC therapy. Multivariate logistic regression analysis was performed to identify the predictive factors for response after IVGC therapy. The OR of the percentage of pretreatment CD3+T cell was 0.718 (95% CI, 0.526 ~ 0.980; P = 0.037) and that of the pretreatment CAS-max in both eyes was 7.221 (95% CI, 1.526 ~ 34.160; P = 0.013). These results suggested that the percentage of pretreatment CD3+T cell and the pretreatment CAS-max in both eyes were independently associated with therapeutic efficacy ().
Table 5. Predictive factors for response after IVGC therapy by binary logistic regression analysis.
The diagnostic accuracy of the percentage of pretreatment CD3+T cell was 75.27% (95% CI, 0.726 ~ 0.960, P = 0.002), based on ROC curve analysis, with an AUCof 0.843, specificity of 86.7% and sensitivity of 87.5%. The diagnostic accuracy of the pretreatment CAS-max in both eyes was 2.5 (95% CI, 0.579 ~ 0.985, P = 0.012), with an AUC of 0.782, specificity of 62.5% and sensitivity of 93.3%. Furthermore, a multivariable prediction model was established with variables of the percentage of pretreatment CD3+T cell and the pretreatment CAS-max in both eyes. The ROC curve analysis showed an AUC of 0.906 (95% CI, 0.783–1.000, p = 0.000), which was better than those single-variable prediction models ().
The Analysis of Predictive Factors (Post-Treatment Clinical Indicators) for Clinical Outcomes of IVGC Therapy
Post-Treatment Clinical Characteristics of Subjects According to Response After IVGC Therapy
As shown in , the percentage of CD19+ B cell was higher in the Group I than in the Group NI (P < 0.05). The level of FT4, the percentage of CD3+T cell and CD3+CD8+T cell were lower in the Group I than in the Group NI (all P < 0.05). Other indicators were not statistically significant between the two groups (all P > 0.05).
Table 6. Post-treatment clinical characteristics of subjects according to response after IVGC therapy.
2-2-2. Predictive factors (post-treatment clinical indicators) for response after IVGC therapy by binary logistic regression analysis.
Factors associated with the efficacy of IVGC therapy were first assessed by univariate analysis. As shown in , the percentage of post-treatment CD3+T cell (P = 0.023), post-treatment CD3+CD8+T cell (P = 0.044), post-treatment CD19+ B cell (P = 0.017) and pretreatment CAS-max in both eyes (P = 0.019) were associated with the efficacy of IVGC therapy. Subsequent multivariate logistic regression analysis showed that pretreatment CAS-max in both eyes (OR = 156.53; 95% CI, 1.725 ~ 14203.14; P = 0.028) and the percentage of post-treatment CD3+T cell (OR = 0.554; 95% CI, 0.313 ~ 0.980; P = 0.043) were independently associated with therapeutic efficacy ().
Table 7. Predictive factors for response after IVGC therapy by binary logistic regression analysis.
The diagnostic accuracy of the percentage of post-treatment CD3+T cell was 63.15% (95% CI, 0.681 ~ 0.925, P = 0.007), based on ROC curve analysis, with an AUC of 0.803, specificity of 60.0% and sensitivity of 100%. The diagnostic accuracy of the pretreatment CAS-max in both eyes was 2.5 (95% CI, 0.579 ~ 0.985, P = 0.012), with an AUC of 0.782, specificity of 62.5% and sensitivity of 93.3%. Furthermore, a multivariable prediction model was established with variables of the percentage of post-treatment CD3+T cell and the pretreatment CAS-max in both eyes. The ROC curve analysis showed an AUC of 0.925 (95% CI, 0.834 ~ 1.000, p = 0.000), which was better than those single-variable prediction models ().
Discussion
The present study aimed to analyze risk factors for activity and predictive factors for clinical outcomes of IVGC therapy in patients with moderate-to-severe TAO. The results of this study indicated that abnormal TRAb and the percentage of CD3+CD4+ T cell were independent risk factors for the activity of moderate-to-severe TAO patients. Moreover, our results showed that the pretreatment CAS-max in both eyes was positive independent correlation with therapeutic efficacy, while the percentage of pretreatment CD3+T cell was negative independent correlation with therapeutic efficacy. And the pretreatment CAS-max in both eyes was positive independent correlation with therapeutic efficacy, while the percentage of post-treatment CD3+T cell was negative independent correlation with therapeutic efficacy.
Risk Factors for Activity of TAO Patients
It is generally accepted that TRAB plays an indispensable role in the pathogenesis of TAO.Citation34 A number of clinical studies have shown that TRAbs correlate with the severity and the clinical activity of TAO.Citation35,Citation36 The results of this study suggested that TAO patients with abnormal TRAb (above the upper limit of normal range) were at greater risk of having active TAO, which was consistent with previous studies. In particular, it has been suggested that TSH-R is likely involved especially in the initial/early stages of TAO, being its expression in orbital fibroadipose tissue correlated with the serum levels of TRAbs, and thus, presumably, with TAO activity.Citation20 Therefore, it is important for clinicians to give TAO patients antithyroid drug therapy timely for keeping the thyroid gland function normal and thus maintain TRAb within the normal range.
As the percentage of CD3+CD4+ T cell increased, the patient was at greater risk of having active TAO. Tyutyunikov et al. found an increased percentage of CD4+ helper T cells in patients with active moderately severe TAO, but not in patients with mild stable TAO.Citation37 It suggested that the severity and activity of TAO may influence the distribution of peripheral T cell subsets. And another study found that CD4+ helper T cells were increased in patients with active TAO.Citation23 CD4+ T cells are the most abundant lymphocytes infiltrating TAO orbital tissues and have been reported to initiate and perpetuate orbital inflammation in TAO.Citation14,Citation38 Orbital fibroblasts, as the target cells in TAO, are specifically activated by the T cell receptor (TCR) on antigen-specific CD4+ T cellsCitation39 and perpetually stimulated by T cells expressing cytokines and chemokines, such as interferon-γ (IFN-γ), tumor necrosis factor and C – X–C motif ligands.Citation40 It is suggested that the Th1 immune response may predominate in early active TAO.Citation41 The above studies are consistent with the results of the present study. In addition, a previous study showed that the percentage of CD3+CD4-CD8- T cells decreased in the active TAO patients and had a negative correlation with the activity of TAO.Citation42 CD3+CD4-CD8- T cells regulate inflammation by suppressing syngeneic CD4+ or CD8+ T cells.Citation43 Recently, CD3+CD4+CD8+ T cells were found in the orbital connective tissue of active TAO patients.Citation44 Currently, little is known about the relationship between T cell subsets and TAO, so future studies with large sample sizes are still needed to investigate the relationship between them.
Besides, a multivariable prediction model was established including variables of age, abnormal TRAb and the percentage of CD3+CD4+ T cell, which was better in the forecasting aspect than other prediction models with an AUC of 0.756. We hope to verify this model in larger cohorts. Hope it can be used to predict TAO activity.
Predictive Factors for Clinical Outcomes of IVGC Therapy in TAO Patients
The higher the pretreatment CAS, the better the effect of IVGC therapy. A higher pretreatment CAS suggests an increased disease activity that is more likely to be attenuated by IVGC therapy.Citation31 As defined by the EUGOGO, a CAS ≥ 3/7 is sufficient for the diagnosis of active TAO.Citation45 However, this criterion was established in Caucasian patients, and previous studies have reported considerably lower rates of eyelid redness present in Asian patients than in Caucasian patients (5.13%–10.26% vs 53.5%),Citation31,Citation46 which might be attributed to the differences in eyelid anatomy between Caucasian and Asian populations.Citation47 This difference makes the cutoff point of CAS for Asian patients likely to be < 3/7. Therefore, in the present study, patients with a CAS < 3/7 but with increased signal intensity of extraocular muscles on contrast-enhanced orbital MRI were also included. Based on the results of ROC analysis, it was found that IVGC therapy was more effective when the CAS was > 2.5 according to the optimal cutoff value (sensitivity of 93.3% and specificity of 62.5%). The present finding is consistent with the results from a previous study also conducted in Asian patients.Citation29 However, large prospective studies in China are required to confirm this finding.
Multivariate logistic regression analysis showed that the percentage of pretreatment CD3+T cell () and the percentage of post-treatment CD3+T cell () were negative independent correlation with therapeutic efficacy, respectively. The lymphocyte-mediated immunity is thought to be the initiating factor of TAO.Citation48 Among these lymphocytes, T cells are thought to be the main effector cells, which produce a variety of adhesion molecules and cytokines, mediate the recruitment of more lymphocytes into orbital tissues, and promote the proliferation and differentiation of OFs.Citation49,Citation50 IVGC are nonspecific in action, exerting powerful anti-inflammatory and immunomodulatory effects on the activity and survival of almost all immune cells.Citation51,Citation52 Based on the results of ROC analysis, it was found that IVGC therapy was more effective when the percentage of pretreatment CD3+T cell was < 75.27% according to the optimal cutoff value (sensitivity of 87.5% and specificity of 86.7%) or the percentage of post-treatment CD3+T cell was < 63.15% according to the optimal cutoff value (sensitivity of 100% and specificity of 60.0%). The decline in the percentage of post-treatment CD3+T cell reflects the resolution of the inflammation. According to the above results, we suggest that the percentage of pretreatment of CD3+T cell could be used as a reference index to predict the effect of IVGC therapy, and clinicians should dynamically monitor the change of it during treatment, which could be helpful for them to timely judge the effect of treatment for TAO patients.
Besides, multivariable prediction models were established including variables of the percentage of pretreatment CD3+T cell and the pretreatment CAS-max in both eyes (AUC = 0.906) or the percentage of post-treatment CD3+T cell and the pretreatment CAS-max in both eyes (AUC = 0.925), which were better in the forecasting aspect than those single-variable prediction models. These models may serve as a useful tool for determining the indication and prognosis of IVGC therapy in clinical practice.
So far, the impact of smoking on IVGC therapy remained controversial. A previous observational study of 92 patients showed that smoking, even passive smoking, was associated with poor therapeutic response to IVGC in TAO patients.Citation53 Whereas another recent retrospective study of 90 patients reported that smoking was not associated with the efficacy of IVGC therapy.Citation29 In this study, we also found no significant correlation between smoking and therapeutic efficacy. A possible reason for the discrepancy could be differences in the way for classifying smoking status. In the observational study, patients were divided into never-smokers, active smokers and passive smokers.Citation53 Another study mentioned above did not take passive smoking into account.Citation29 In the present study, we classified patients as never-smokers and smokers (active smokers and passive smokers). Therefore, future studies with large sample sizes are still needed to investigate the impact of different smoking status on the efficacy of IVGC therapy in TAO patients.
One problem of this trial was that two IVGC treatment regimens were used. However, the total amount of both schemes was 4.5 g of intravenous methylprednisolone. Firstly, there was no difference in the use of the two regimens between Group I and Group NI (). Secondly, the multivariable prediction model showed similarly good discrimination and calibration in the two groups ().
There were still some limitations. Firstly, because this study was conducted in a tertiary hospital, there may be a selection bias in patient selection. The present study involved only patients with moderate-to-severe TAO and probably the described risk factors for activity was not fully representative for TAO in general. Secondly, it is a small sample retrospective study, so our results need to be replicated in prospective research with a large sample size. Finally, our research is a clinical study which does not involve the function of T cell, and much fewer mechanisms. Future animal or human experiments are required to clarify the potential function and mechanism in the future.
Conclusion
In conclusion, it can be seen from above results that the monitoring of peripheral blood T cell subsets is of great significance for TAO patients in clinical work, which could be helpful to timely judge the condition of clinical features and effect of treatment for TAO patients. Moreover, it was found that IVGC therapy was more effective when the CAS was > 2.5. Therefore, the identification of modifiable predictors can provide practical intervention targets for the management of patients with TAO to improve treatment outcomes. Multivariable prediction models have been established, which have great significance for clinical work. However, further prospective studies are required to verify these findings.
Author Contributions
H.L. and B.W. wrote the manuscript. Y.L., B.H., and H.Z. designed the research. H.L., B.W., Qin Li, Qing Li, J.Q., D.L., C.S., L.Y., H.Z., B.J., N.W., B.H., M.J., X.T., Z.S., C.Z., Y.M., P.X., and J.S. performed the research. H.L., B.W., and Qin Li. analyzed the data. H.L, Y.L., and H.Z. contributed new reagents/analytical tools.
Acknowledgments
The authors thank all members of the Multidisciplinary team (MDT) and all participants in the study.
Disclosure Statement
No potential conflict of interest was reported by the author(s).
Data Availability Statement
The data supporting the findings of this study are available on reasonable request from the corresponding authors.
Additional information
Funding
References
- Campi I, Vannucchi G, Muller I, Lazzaroni E, Currò N, Dainese M, et al. Therapy with different dose regimens of rituximab in patients with active moderate-to-severe graves’ orbitopathy. Front Endocrinol (Lausanne). 2022;12:790246. doi:10.3389/fendo.2021.790246.
- Bartalena L, Pinchera A, Marcocci C. Management of Graves’ ophthalmopathy: reality and perspectives. Endocr Rev. 2000;21(2):168–199. doi:10.1210/edrv.21.2.0393.
- Burch HB, Wartofsky L. Graves’ ophthalmopathy: current concepts regarding pathogenesis and management. Endocr Rev. 1993;14(6):747–793. doi:10.1210/edrv-14-6-747.
- Hiromatsu Y, Eguchi H, Tani J, Kasaoka M, Teshima Y. Graves’ ophthalmopathy: epidemiology and natural history. Intern Med. 2014;53(5):353–360. doi:10.2169/internalmedicine.53.1518.
- Lazarus JH. Epidemiology of Graves’ orbitopathy (GO) and relationship with thyroid disease. Best Pract Res Clin Endocrinol Metab. 2012;26(3):273–279. doi:10.1016/j.beem.2011.10.005.
- Bartalena L, Tanda ML. Clinical practice. Graves’ ophthalmopathy. N Engl J Med. 2009;360(10):994–1001. doi:10.1056/NEJMcp0806317.
- Wang Y, Smith TJ. Current concepts in the molecular pathogenesis of thyroid-associated ophthalmopathy. Invest Ophthalmol Vis Sci. 2014;55(3):1735–1748. doi:10.1167/iovs.14-14002.
- Smith TJ, Hegedüs LG. Disease. N Engl J Med. 2016;375(16):1552–1565. doi:10.1056/NEJMra1510030.
- Wiersinga WM. Advances in treatment of active, moderate-to-severe Graves’ ophthalmopathy. Lancet Diabetes Endocrinol. 2017;5(2):134–142. doi:10.1016/s2213-8587(16)30046-8.
- Davies TF, Andersen S, Latif R, Nagayama Y, Barbesino G, Brito M, et al. Graves’ disease. Nat Rev Dis Primers. 2020;6(1):52. doi:10.1038/s41572-020-0184-y.
- Smith TJ. Pathogenesis of Graves’ orbitopathy: a 2010 update. J Endocrinol Invest. 2010;33(6):414–421. doi:10.1007/bf03346614.
- Karoutsou E, Polymeris A. Pathogenesis of Graves’ disease focusing on Graves’ ophthalmopathy. Endocr Regul. 2011;45(4):209–220. doi:10.4149/endo_2011_04_209.
- Salvi M. Immunotherapy for Graves’ ophthalmopathy. Curr Opin Endocrinol Diabetes Obes. 2014;21(5):409–414. doi:10.1097/med.0000000000000097.
- Khong JJ, McNab AA, Ebeling PR, Craig JE, Selva D. Pathogenesis of thyroid eye disease: review and update on molecular mechanisms. Br J Ophthalmol. 2016;100(1):142–150. doi:10.1136/bjophthalmol-2015-307399.
- Li H, Wang T. The autoimmunity in Graves’s disease. Front Biosci (Landmark Ed). 2013;18(2):782–787. doi:10.2741/4141.
- Gianoukakis AG. Smith TJ.Recent insights into the pathogenesis and management of thyroid-associated ophthalmopathy. Curr Opin Endocrinol Diabetes Obes. 2008;15(5):446–452. doi:10.1097/MED.0b013e32830eb8ab.
- Pawlowski P, Reszec J, Eckstein A, Johnson K, Grzybowski A, Chyczewski L, et al. Markers of inflammation and fibrosis in the orbital fat/connective tissue of patients with Graves’ orbitopathy: clinical implications. Mediators Inflamm. 2014;412158. doi:10.1155/2014/412158.
- Virakul S, van Steensel L, Dalm VA, Paridaens D, van Hagen PM, Dik WA. Platelet-derived growth factor: a key factor in the pathogenesis of graves’ ophthalmopathy and potential target for treatment. Eur Thyroid J. 2014;3(4):217–226. doi:10.1159/000367968.
- Hwang CJ, Afifiyan N, Sand D, Naik V, Said J, Pollock SJ, et al. Orbital fibroblasts from patients with thyroid-associated ophthalmopathy overexpress CD40: cD154 hyperinduces IL-6, IL-8, and MCP-1. Invest Ophthalmol Vis Sci. 2009;50(5):2262–2268. doi:10.1167/iovs.08-2328.
- Hai YP, Lee ACH, Frommer L, Diana T, Kahaly GJ. Immunohistochemical analysis of human orbital tissue in Graves’ orbitopathy. J Endocrinol Invest. 2020;43(2):123–137. doi:10.1007/s40618-019-01116-4.
- Grubeck-Loebenstein B, Trieb K, Sztankay A, Holter W, Anderl H, Wick G. Retrobulbar T cells from patients with Graves’ ophthalmopathy are CD8+ and specifically recognize autologous fibroblasts. J Clin Invest. 1994;93(6):2738–2743. doi:10.1172/JCI117289.
- Rotondo Dottore G, Torregrossa L, Caturegli P, Ionni I, Sframeli A, Sabini E, et al. Association of T and B cells infiltrating orbital tissues with clinical features of graves orbitopathy. JAMA Ophthalmol. 2018;136(6):613–619. doi:10.1001/jamaophthalmol.2018.0806.
- Vaidya B, Shenton BK, Stamp S, Miller M, Baister E, Andrews CD, et al. Analysis of peripheral blood T-cell subsets in active thyroid-associated ophthalmopathy: absence of effect of octreotide-LAR on T-cell subsets in patients with thyroid-associated ophthalmopathy. Thyroid. 2005;15(9):1073–1078. doi:10.1089/thy.2005.15.1073.
- Smith TJ, Kahaly GJ, Ezra DG, Fleming JC, Dailey RA, Tang RA, et al. Teprotumumab for Thyroid-Associated Ophthalmopathy. N Engl J Med. 2017;376(18):1748–1761. doi:10.1056/NEJMoa1614949.
- Genere N, Stan MN. Current and emerging treatment strategies for Graves’ Orbitopathy. Drugs. 2019;79(2):109–124. doi:10.1007/s40265-018-1045-9.
- Taylor PN, Zhang L, Lee RWJ, Muller I, Ezra DG, Dayan CM, et al. New insights into the pathogenesis and nonsurgical management of Graves orbitopathy. Nat Rev Endocrinol. 2020;16(2):104–116. doi:10.1038/s41574-019-0305-4.
- Bartley GB. Gorman CA.Diagnostic criteria for Graves’ ophthalmopathy. Am J Ophthalmol. 1995;119(6):792–795. doi:10.1016/s0002-9394(14)72787-4.
- Bartalena L, Baldeschi L, Dickinson A, Eckstein A, Kendall-Taylor P, Marcocci C, et al. Consensus statement of the European Group on Graves’ orbitopathy (EUGOGO) on management of GO. Eur J Endocrinol. 2008;158(3):273–285. doi:10.1530/eje-07-0666.
- Wang Y, Zhang S, Zhang Y, Liu X, Gu H, Zhong S, et al. A single-center retrospective study of factors related to the effects of intravenous glucocorticoid therapy in moderate-to-severe and active thyroid-associated ophthalmopathy. BMC Endocr Disord. 2018;18(1):13. doi:10.1186/s12902-018-0240-8.
- Zang S, Ponto KA, Kahaly GJ. Clinical review: intravenous glucocorticoids for Graves’ orbitopathy: efficacy and morbidity. J Clin Endocrinol Metab. 2011;96(2):320–332. doi:10.1210/jc.2010-1962.
- Mourits MP, Prummel MF, Wiersinga WM, Koornneef L. Clinical activity score as a guide in the management of patients with Graves’ ophthalmopathy. Clin Endocrinol (Oxf). 1997;47(1):9–14. doi:10.1046/j.1365-2265.1997.2331047.x.
- Naselli A, Moretti D, Regalbuto C, Arpi ML, Lo Giudice F, Frasca F, et al. Evidence that baseline levels of low-density lipoproteins cholesterol affect the clinical response of Graves’ ophthalmopathy to parenteral corticosteroids. Front Endocrinol (Lausanne). 2020;11:609895. doi:10.3389/fendo.2020.609895.
- Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32–35. doi:10.1002/1097-0142(1950)3:1<32:AID-CNCR2820030106>3.0.CO;2-3.
- Khoo TK, Bahn RS. Pathogenesis of Graves’ ophthalmopathy: the role of autoantibodies. Thyroid. 2007;17(10):1013–1018. doi:10.1089/thy.2007.0185.
- Gerding MN, van der Meer JW, Broenink M, Bakker O, Wiersinga WM, Prummel MF. Association of thyrotrophin receptor antibodies with the clinical features of Graves’ ophthalmopathy. Clin Endocrinol (Oxf). 2000;52(3):267–271. doi:10.1046/j.1365-2265.2000.00959.x.
- Lytton SD, Ponto KA, Kanitz M, Matheis N, Kohn LD, Kahaly GJ. A novel thyroid stimulating immunoglobulin bioassay is a functional indicator of activity and severity of Graves’ orbitopathy. J Clin Endocrinol Metab. 2010;95(5):2123–2131. doi:10.1210/jc.2009-2470.
- Tyutyunikov A, Raikow RB, Kennerdell JS, Kazim M, Dalbow MH, Scalise D. Re-examination of peripheral blood T cell subsets in dysthyroid orbitopathy. Invest Ophthalmol Vis Sci. 1992;33(7):2299–2303. PMID: 1351477.
- Smith TJ. TSH-receptor-expressing fibrocytes and thyroid-associated ophthalmopathy. Nat Rev Endocrinol. 2015;11(3):171–181. doi:10.1038/nrendo.2014.226.
- Otto EA, Ochs K, Hansen C, Wall JR, Kahaly GJ. Orbital tissue-derived T lymphocytes from patients with Graves’ ophthalmopathy recognize autologous orbital antigens. J Clin Endocrinol Metab. 1996;81(8):3045–3050. doi:10.1210/jcem.81.8.8768872.
- Antonelli A, Ferrari SM, Fallahi P, Frascerra S, Santini E, Franceschini SS, et al. Monokine induced by interferon gamma (IFNgamma) (CXCL9) and IFNgamma inducible T-cell alpha-chemoattractant (CXCL11) involvement in Graves’ disease and ophthalmopathy: modulation by peroxisome proliferator-activated receptor-gamma agonists. J Clin Endocrinol Metab. 2009;94(5):1803–1809. doi:10.1210/jc.2008-2450.
- Aniszewski JP, Valyasevi RW, Bahn RS. Relationship between disease duration and predominant orbital T cell subset in Graves’ ophthalmopathy. J Clin Endocrinol Metab. 2000;85(2):776–780. doi:10.1210/jcem.85.2.6333.
- Hu H, Liang L, Ge Q, Jiang X, Fu Z, Liu C, et al. Correlation between peripheral t cell subsets and the activity of thyroid-associated ophthalmopathy. Int J Endocrinol. 2022;2022:2705650. doi:10.1155/2022/2705650.
- Chen W, Ford MS, Young KJ, Zhang L. The role and mechanisms of double negative regulatory T cells in the suppression of immune responses. Cell Mol Immunol. 2004;1(5):328–335. PMID: 16285891.
- Xu D, Wu Y, Gao C, Qin Y, Zhao X, Liang Z, et al. Characteristics of and reference ranges for peripheral blood lymphocytes and CD4+ T cell subsets in healthy adults in Shanxi province, North China. J Int Med Res. 2020;48(7):1–13. doi:10.1177/0300060520913149.
- Bartalena L, Baldeschi L, Boboridis K, Eckstein A, Kahaly GJ, Marcocci C, et al. The 2016 European thyroid association/European group on Graves’ Orbitopathy guidelines for the Management of Graves’ Orbitopathy. Eur Thyroid J. 2016;5(1):9–26. doi:10.1159/000443828.
- Zhu W, Ye L, Shen L, Jiao Q, Huang F, Han R, et al. A prospective, randomized trial of intravenous glucocorticoids therapy with different protocols for patients with graves’ ophthalmopathy. J Clin Endocrinol Metab. 2014;99(6):1999–2007. doi:10.1210/jc.2013-3919.
- Jeong S, Lemke BN, Dortzbach RK, Park YG, Kang HK. The Asian upper eyelid: an anatomical study with comparison to the Caucasian eyelid. Arch Ophthalmol. 1999;117(7):907–912. doi:10.1001/archopht.117.7.907.
- Huang Y, Fang S, Li D, Zhou H, Li B. Fan X.The involvement of T cell pathogenesis in thyroid-associated ophthalmopathy. Eye (Lond). 2019;33(2):176–182. doi:10.1038/s41433-018-0279-9.
- Pappa A, Lawson JM, Calder V, Fells P, Lightman S. T cells and fibroblasts in affected extraocular muscles in early and late thyroid associated ophthalmopathy. Br J Ophthalmol. 2000;84(5):517–522. doi:10.1136/bjo.84.5.517.
- Fang S, Huang Y, Wang N, Zhang S, Zhong S, Li Y, et al. Insights into local orbital immunity: Evidence for the involvement of the Th17 cell pathway in thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 2019;104(5):1697–1711. doi:10.1210/jc.2018-01626.
- Cari L, De Rosa F, Nocentini G, Riccardi C. Context-dependent effect of glucocorticoids on the proliferation, differentiation, and apoptosis of regulatory t cells: A review of the empirical evidence and clinical applications. Int J Mol Sci. 2019;20(5). doi:10.3390/ijms20051142.
- Strehl C, Ehlers L, Gaber T. Buttgereit F.Glucocorticoids-all-rounders tackling the versatile players of the immune system. Front Immunol. 2019;10:1744. doi:10.3389/fimmu.2019.01744.
- Xing L, Ye L, Zhu W, Shen L, Huang F, Jiao Q, et al. Smoking was associated with poor response to intravenous steroids therapy in Graves’ ophthalmopathy. Br J Ophthalmol. 2015;99(12):1686–1691. doi:10.1136/bjophthalmol-2014-306463.