Abstract
End-stage renal disease (ESRD) coexisted with cirrhosis, ascites, and primary liver cancer represents an extraordinarily rare clinical condition that typically occurs in very late-stage decompensated cirrhosis and is associated with an extremely poor prognosis. We present a case of a 68-year-old male patient with ESRD who experienced various decompensated complications of liver cirrhosis, particularly massive ascites and hepatic space-occupying lesions. Peritoneal dialysis (PD) catheter insertion and continuous ambulatory peritoneal dialysis (CAPD) treatment were successfully performed. During meticulous follow-up, the patient survived for one year but ultimately succumbed to complications related to liver cancer. PD can serve as an efficacious therapeutic approach for such late-stage patients afflicted together with severe cirrhosis, massive ascites and primary liver cancer.
There has been an increasing incidence of chronic kidney disease (CKD) in patients with liver cirrhosis in recent years [Citation1]. Epidemiological data indicate that the average survival time for individuals with decompensated cirrhosis is typically 3–5 years, and most CKD patients with decompensated cirrhosis succumb to complications before reaching end-stage renal disease (ESRD) [Citation2]. Therefore, the reports on cirrhosis complicated with ESRD are extremely limited, the choice of renal replacement therapy (RRT) for such patients remains a subject of controversy. Currently, hemodialysis (HD) continues to be the primary treatment in the majority of cases [Citation3]. Liver cancer is a manifestation of advanced cirrhosis. There are few reports on peritoneal dialysis (PD) treatment for ESRD patients complicated with liver cancer [Citation4–8]. Furthermore, no instances have been reported where PD treatment was chosen upon early detection of signs indicating liver cancer. Here, we reported a successful implementation of continuous ambulatory peritoneal dialysis (CAPD) in a patient presenting with cirrhosis, massive ascites and a liver space-occupying lesion.
A 68-year-old man was admitted to the Nephrology department on March 4th, 2021 with severe abdominal distension, lower limb edema, nausea and vomiting for about 1 month. He was diagnosed with ESRD for 10 months and had a 15-year history of schistosomiasis cirrhosis. A 29 × 20mm space-occupying lesion in the right lobe of the liver was detected on May 20, 2020, and close imaging monitoring was performed approximately every 3 months thereafter. As the patient had been treated for cirrhosis complications in our hospital for many years, his entire clinical course was fully recorded (). The specific test results obtained during the follow-up are presented in Supplementary Table 1. In addition, the patient had no prior history of infection or familial inheritance, and his personal medical history was unremarkable. Physical examination showed that the patient had chronic facial features, eyelid edema, palpebral conjunctiva pallor, skin without yellow staining, and exhaled gas with urea smell. Both upper limbs were slim, subcutaneous fat content was low, and blood pressure was 134/83 mmHg. Cardiopulmonary examination showed no significant abnormalities. The abdomen was significantly distended, exhibiting varicose veins in the abdominal wall. There was no tenderness or rebound pain. Pitted edema could be observed in both lower limbs. Laboratory tests revealed the following results: Alanine aminotransferase level was 8 U/L, aspartate aminotransferase level was 5 U/L, albumin level was 33.6 g/L, urea nitrogen level was elevated at 43.5 mmol/L, creatinine (picric acid method) level showed an increase of 688.5 μmol/L, glomerular filtration rate was decreased to 6.44 mL/(min/1.73m2), Parathyroid hormone concentration measured at 123.7 pg/mL, hemoglobin level was reduced to 62 g/L, platelet count showed a decrease to 50 G/L; urine analysis revealed a volume of 900 mL in a span of 24 h with protein presence graded as 2+ and total protein excretion measuring at 608 mg/24h; stool occult blood test result was positive (+). The patient’s Child-Pugh rating was classified as grade B. The abdominal CT examination revealed a decrease in liver volume with irregularly proportioned lobes and an enlarged hepatic portal vein measuring approximately 18 mm in diameter. Additionally, there was observed splenic vein enlargement along with thickening and twisting of the gastric fundus esophageal vein. The umbilical vein remained open, while an enlarged spleen was present accompanied by abdominal fluid accumulation. A slightly hypoechoic mass measuring about 34 × 35mm in size was detected adjacent to the liver’s right lobe capsule, displaying well-defined boundaries.
Figure 1. The entire clinical course of the patient. Light-blue color box represents the clinical course after PD catheter insertion. WBC: White blood cell, Hb: Hemoglobin, PLT: Platelet, BUN: Blood urine nitrogen, Alb: Albumin, Scr: Serum creatinine.
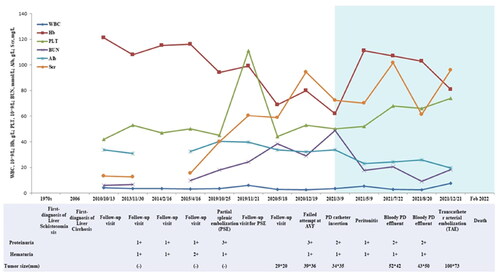
A straight, double-cuffed PD catheter (Tenckhoff type) was surgically implanted under local anesthesia on March 11th, 2021. When the catheter was placed, about 1000 mL of amber-colored peritoneal fluid was evacuated. After 2 weeks, the patient successfully transitioned from intermittent peritoneal dialysis (IPD) to CAPD. No PD-related complications occurred during treatment. The patient was regularly followed up outside the hospital (). On May 9th, 2021, he was admitted to the hospital due to abdominal pain and cloudy effluent for one day. The routine results of effluent revealed a total leukocyte count of 931 × 106/L, with polynuclear leukocytes accounting for 80%. The diagnosis was PD-related peritonitis. Effluent culture results indicated an infection caused by salivary streptococcus. Based on drug sensitivity findings, amikacin sulfate per night and vancomycin hydrochloride per week were administered, leading to the patient’s recovery from peritonitis. Subsequently, on July 17th, 2021 and August 19th, 2021, the patient was readmitted due to hemorrhagic effluents. Examination results showed positive fecal occult blood test findings. Considering severe portal hypertension vascular complications and gastrointestinal bleeding, the patient received treatments with proton pump inhibitors (PPIs), octreotide acetate infusion pumps, hemostatic agents (phenolxanthamine), and vitamin K1 supplementation. The effluents gradually became clear and the patient was discharged. CAPD maintenance therapy was continued outside the hospital. Simultaneously, drug therapy for liver protection, kidney protection, anti-hypertension (dose adjustment with close monitoring of blood pressure), and improvement of anemia was administered. On December 19th, 2021, the patient presented to our emergency department complaining of right upper abdominal pain persisting for six days. CT scan revealed significant enlargement of liver mass measuring approximately 100 × 73mm, and considering alpha-fetoprotein (AFP) level as high as 227.l U/L, a malignant tumor lesion could be suspected. Transcatheter arterial embolization (TAE) was subsequently performed. During the entire disease course, no liver biopsy or surgical resection for occupying lesions had been conducted due to the patients’ physical limitations and personal willingness. Unfortunately, the patient died from complications of liver cancer in February 2022.
During the follow-up period, the patient experienced a single episode of cloudy PD effluent, PD effluent culture revealed Streptococcus salivarius as the pathogenic bacterium, which differs from typical cases of spontaneous peritonitis. Furthermore, it occurred during maintenance CAPD, suggesting a high probability that improper operation during PD treatment was responsible for its onset. Subsequently, the patient experienced bloody effluent twice but showed improvement following proton pump inhibitor therapy and hemostasis treatments-indicating mild abdominal bleeding, which holds little clinical significance.
In addition, we reviewed other similar reports to explore the potentialities and experiences of CAPD application in these patients (). In particular, the PD treatment was initiated in our case after the appearance of early signs of liver cancer. Furthermore, the patient has been regularly followed up for nearly 20 years, resulting in comprehensive records detailing the etiology, complications, and treatment of ESRD that may be difficult to capture in other medical record reports. Our practice suggests that PD can be considered as an effective and feasible renal replacement therapy in patients with cirrhosis, massive ascites and primary liver cancer. The treatment process, however, necessitates diligent monitoring and follow-up to ensure meticulous nursing care is provided and minimize the risk of infection resulting from improper procedures. The clinical experience garnered from this particular case could be shared to other medical facilities for treating the similar patients.
Table 1. Summary of peritoneal dialysis in ESRD patients with primary liver cancer.
Ethical approval
This study was approved by the Institutional Review Board of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology.
Supplemental Material
Download MS Word (22 KB)Acknowledgments
The authors thank our patient for allowing for his case to be presented.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Kumar R, Priyadarshi RN, Anand U. Chronic renal dysfunction in cirrhosis: a new frontier in hepatology. World J Gastroenterol. 2021;27(11):1–4. doi: 10.3748/wjg.v27.i11.990.
- Tapper EB, Parikh ND. Diagnosis and management of cirrhosis and its complications a review. Jama-J Am Med Assoc. 2023;329(18):1589–1602. doi: 10.1001/jama.2023.5997.
- Nader MA, Aguilar R, Sharma P, et al. In-hospital mortality in cirrhotic patients with end-stage renal disease treated with hemodialysis versus peritoneal dialysis: a nationwide study. Perit Dial Int. 2017;37(4):464–471. doi: 10.3747/pdi.2016.00131.
- Dozio B, Scanziani R, Rovere G, et al. Hemoperitoneum in a continuous ambulatory peritoneal dialysis patient caused by a hepatocarcinoma treated with percutaneous embolization. Am J Kidney Dis. 2001;38(3):E11.
- Peng SJ, Yang CS. Hemoperitoneum in CAPD patients with hepatic tumors. Perit Dial Int. 1996;16(1):84–85. doi: 10.1177/089686089601600121.
- Lui SL, Yip PS, Lam MF, et al. Feasibility of reinstitution of CAPD after partial hepatectomy in patients with malignant hepatic tumors. Perit Dial Int. 2003;23(5):504–506. doi: 10.1177/089686080302300519.
- Posthuma N, van Eps RS, ter Wee PM. Hemoperitoneum due to (hepatocellular) adenoma. Perit Dial Int. 1998;18(4):446–447. doi: 10.1177/089686089801800421.
- Boyer A, Jegonday MA, Lanot A, et al. Percutaneous ablation for hepatocellular carcinoma and peritoneal dialysis. Perit Dial Int. 2017;37(6):656–658. +. doi: 10.3747/pdi.2017.00073.

