Abstract
Background A growing challenge in the opioid epidemic is the rise of highly potent synthetic opioids, (i.e., illicitly manufactured fentanyl [IMF]) entering the US non-prescription opioid market. Successful reversal may require multiple doses of naloxone, the standard of care for opioid overdose. We conducted a narrative literature review to summarize the rates of multiple naloxone administrations (MNA) for opioid overdose reversal. Methods: A MEDLINE search was conducted for published articles using MESH search terms: opioid overdose, naloxone and multiple naloxone administration. Of the 2,101 studies identified, articles meeting inclusion/exclusion criteria were reviewed, categorized by primary and secondary outcomes of interest and summarized by data source and study design. Results: A total of 24 articles meeting eligibility criteria were included. Among EMS-based studies, MNA rates ranged from 9% to 53%; in general, bystander-reported studies were notably higher, from 16% to 89%. Variation in study design, data sources, year and geography, may have contributed to these ranges. Three studies that included longitudinal results reported a significant percent increase between 26% and 43% in annual MNA rates or a significant increase in mean naloxone doses over time (p < .001). Conclusions: This summary found that multiple naloxone administrations during opioid overdose encounters vary widely, have occurred in up to 89% of all opioid overdoses, and have significantly increased over time. Higher naloxone formulations may fulfill an unmet need in opioid overdose reversals, given the rising rates of overdoses involving IMF. Further studies are needed to gain a better understanding of MNA during opioid overdose encounters, particularly across a wider geographic region in the US in order to inform continuing efforts to combat the opioid epidemic.
Introduction
The opioid overdose epidemic remains a significant public health issue and continues to rise exponentially in the US. In 2017, there were 47,600 deaths due to opioid overdose, which represented 68% of all drug overdose deaths in the US.Citation1 In 2018, over 10 million individuals aged 12 or older (3.7%) in the US reported opioid misuse.Citation2 Recently, a new challenge has emerged—the introduction of synthetic opioids, including fentanyl, analogues such as carfentanil and precursor chemicals into the market. These synthetic opioids are highly powerful, with fentanyl and carfentanil estimated to be up to 100 and 10,000 times more potent than morphine, respectively.Citation3 While fentanyl has been available in prescription form as an analgesic for some time, data indicates that the dramatic rise in drug overdose deaths in recent years can be attributed primarily to the influx of illicitly manufactured synthetic opioids.Citation4,Citation5 The rate of US overdose deaths due to synthetic opioids increased from 9.0 per 100,000 population in 2017 to 9.9 in 2018, with 2 out of 3 opioid-related deaths involving synthetic opioids.Citation6,Citation7 Data from 2013 to 2017 show the average annual percent increase was 69.8% in drug overdose deaths in the US due to synthetic opioids. This represents a marked increase compared to the average annual percent increase of 8.4% from 1999 to 2013.Citation8 The most recent published estimates from January to June 2019 revealed that 61.5% of US drug overdose deaths were due to illicitly manufactured fentanyl (IMF), suggesting that the problem remains unabated.Citation8
Additionally, the COVID-19 pandemic has further exacerbated the opioid overdose epidemic; recent data suggests an increase in non-prescription opioid use during the pandemic with the percent of tested individuals screening positive for IMF and heroin increasing by 35% and 44%, respectively.Citation9
Approved by the FDA in 1971, naloxone is considered the standard of care and has been shown to be effective in opioid overdose reversals.Citation10,Citation11 A key prevention strategy endorsed by the Centers for Disease Control and Prevention (CDC) and the American Medical Association (AMA) is to increase the distribution of and access to naloxone among bystanders, allowing bystanders to administer naloxone to reverse the overdose.Citation12,Citation13 Since 1996, community-based harm reduction programs in the US have distributed naloxone directly to people who use opioids, and other bystanders.Citation14 A recent analysis of data from the first half of 2019 found that nearly 40% of opioid and stimulant overdose deaths occurred in the presence of a bystander,Citation15 thus take-home naloxone can be a critical, life-saving tool.
With the increase of synthetic opioids and the rapid onset of effect, evidence is emerging suggesting the need for increased doses of naloxone to reverse opioid toxicity. Numerous studies have been published examining the occurrence of multiple naloxone administrations (MNA) in opioid overdose reversals,Citation16–30 however, these studies have varied in study design, scientific rigor, sample size and geographic locations, making it difficult to obtain a comprehensive understanding of the frequency and trends of MNA occurrence in the US.
To our knowledge, a summary of the literature in this area has not been published. Thus, the purpose of this paper was to review the existing literature assessing the rates of multiple naloxone administrations for opioid overdose reversal to better understand the circumstances and context in which MNA have occurred. We sought to identify whether a stronger dose formulation of naloxone is needed and possible opportunities to improve the initial reversal response/outcomes for individuals experiencing an opioid overdose.
Methods
A narrative literature review was conducted to summarize published studies examining the rates of multiple administrations of naloxone for the reversal of opioid overdoses. A narrative review methodology was applied due to the dearth of published studies available in this area. Accordingly, results of this review were summarized qualitatively rather than applying meta-analytic summarization techniques.
Search strategy and article selection
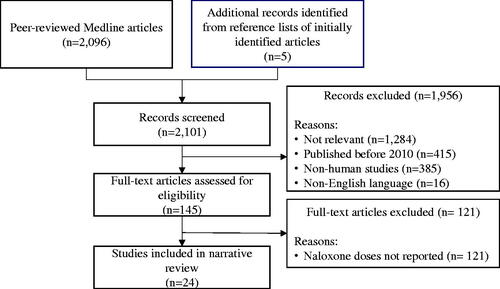
A search was conducted in MEDLINE for peer-reviewed, original research articles published using the following MESH search terms: opioid overdose AND naloxone OR multiple naloxone administration. This initial search yielded 2,096 articles. Five additional papers were identified from the reference lists of the initially identified articles for a total of 2,101 articles. One reviewer reviewed all records and excluded 1,956 articles for the following reasons: studies not relevant to the topic (e.g., pharmacokinetic studies, clinical trials, treatments other than naloxone administered, opioid overdose detection tools, etc.) (n = 1,284), studies published before 2010 (n = 415), non-human studies (n = 385), and non-English language articles (n = 16). Full text articles were then assessed for eligibility. Studies that did not report either the number of naloxone doses administered or the total naloxone dose administered were excluded (n = 121). Due to the small number of available papers, case series and case study articles were included in this review. See for the flow chart depicting the article selection process.
Article abstraction
In accordance with the STROBE (Strengthening Reporting of Observational Studies in Epidemiology) guidelines, the following information from each article was entered into an evidence table to facilitate summarization: author/year, study design, analysis timeframe, sample size, study population, occurrence of multiple naloxone administrations (primary outcome of interest) and total dose administered (secondary outcome of interest) (see ). Additionally, details regarding the rationale, objectives, key results, study limitations and generalizability of each article was documented and analyzed as part of the review process.
Table 1. Summary table of published studies on MNA.
Results
A total of 24 articles met eligibility criteria and were included in this review. The articles were categorized by primary and secondary outcomes of interest and presented in order of data source and study design quality.
Proportion receiving ≥2 doses of naloxone—EMS
depicts the proportion of study participants who received ≥2 doses of naloxone by year of data collection for 13 of the articles that reported this metric with sample sizes greater than 20. Of these 13 studies, 6 were based on emergency medical services (EMS) or EMS and Emergency Department (ED) data, while 7 were based on bystander reported data. Two of the largest studies were retrospective analyses of the National Emergency Medical Service Information Systems (NEMSIS) database,Citation16,Citation17 which contains pre-hospital data collected from EMS encounters across the US. Faul (2017) examined the proportion of opioid overdose events where ≥2 doses of naloxone were administered out of the total number of opioid overdose events where naloxone was used in the pre-hospital setting from 2012 to 2015. The findings showed a significant increase in the proportion of overdose events where ≥2 doses of naloxone were administered over time, with 14.5%, 14.9%, 16.3%, 18.2% from years 2012, 2013, 2014 and 2015, respectively. In this sample, nearly 1 out of every 5 patients received MNA to reverse the overdose in 2015.
Figure 2. Proportion of individuals receiving ≥2 naloxone doses for opioid overdose reversal by study.
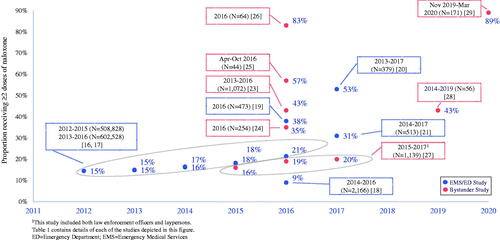
A similar study by GeigerCitation17 utilizing the same database measured the same outcome of ≥2 doses of naloxone administered out of the total number of opioid overdose events in the pre-hospital setting from years 2013 to 2016.Citation17 Since the analysis timeframe of this study overlapped with Faul,Citation16 nearly identical percentages of the proportion of opioid overdose events where ≥2 naloxone doses were administered were found from years 2013 to 2015. In 2016, the proportion was 21.4%, representing a significant increase over 18.1% from 2015. Both the FaulCitation16 and GeigerCitation17 studies were strengthened by having very large sample sizes representing EMS services in all regions within the US.
The above 2 NEMSIS studies were the only ones in this review that encompassed the entire US. However, the data did not include the administration route or the specific naloxone dose. Neither of these studies stratified the MNA rates by region, which would likely show significant variation, given the high incidence of synthetic opioid dispersion in certain regions of the country relative to others. By showing only the overall US rates, it is likely an underestimate of the true MNA occurrence in certain regions. Moreover, neither of these studies included prior administration of naloxone by a bystander in the calculation of the proportion receiving ≥2 doses of naloxone. While the proportion of individuals in both studies reported to have received naloxone from a bystander prior to EMS administration were small (<2%), this may be underreported, as EMS providers may not always be aware of, or record bystander interventions that occurred before their arrival.
The remaining studies that reported MNA for overdose reversals were conducted within limited geographic areas. An EMS-based study from a large EMS provider in New Jersey examined 2,166 adults who received naloxone for suspected opioid overdose from first responders between April 2014 to June 2016 and found the majority of cases (91%) were completely resolved after a single dose of intranasal naloxone. However, 142 (6.6%) did receive 2 doses of naloxone after lack of reversal from 1 dose, and 53 (2.4%) required a 3rd dose.Citation18
Three additional retrospective studies that utilized both EMS and ED data from either urban or suburban tertiary care centers reported the proportion receiving MNA in both the prehospital and ED setting after a suspected opioid overdose. Fidacaro et al, 2019 reported that in January through December 2016, 178 of 473 (38%) patients received MNA in the prehospital setting and approximately 33 (7%) received an additional dose in the ED setting.Citation19 In another study which utilized data from EMS and 2 urban ED care centers between January 2013 to December 2017, 199 of 379 (53%) patients received ≥2 doses of naloxone.Citation20 Further, 91 (16%) were administered 3 doses while 49 (13%) were administered ≥4 doses for reversal. Finally, the third study utilizing EMS and ED data from a suburban academic tertiary care center between 2014 to 2017 found 160 of 513 (31%) patients received ≥2 naloxone administrations in the prehospital setting, while 41 (54%) received multiple doses in the ED.Citation21
It is noted that in each of these combined EMS/ED studies, sample sizes were relatively modest, participants were treated at either 1 or 2 tertiary care centers representing a relatively confined geographic area and generally did not include those who refused transport to the hospital upon ambulance arrival, thereby potentially limiting generalizability. It is also notable that MNA rates in studies utilizing EMS or combined EMS/ED data were generally low relative to bystander-based MNA rates in this review. EMS-based data may be reporting artificially lower MNA rates compared to other sources due to differing EMS guidelines for opioid overdose treatment by state or lack of reliable data on interventions performed prior to, or instead of EMS providers.
Proportion receiving ≥2 doses of naloxone—bystanders
Seven published studies reported the frequency of MNA based on self-reported data from bystanders who witnessed, administered or received naloxone for reversal of an opioid overdose event.Citation23–29 These studies collected data from 2013 up to 2020, thus provided more recent estimates of MNA and included laypersons who were often the first to administer naloxone.
One study that collected data as early as 2013 was based on self-reported MNA from individuals who participated in Prevention Point Pittsburgh (PPP), a community syringe access program that provides naloxone to people at risk for overdose.Citation23 Of the 1,072 opioid overdose reversals reported to PPP across years 2013 to 2016, 462 (43%) required ≥2 doses of naloxone.
Another study collected data from first responder and community-based organizations on naloxone administered in 261 overdose reversal events that occurred in from April to August 2016. Of the 254 overdose events that reported the number of naloxone doses administered, 35% required ≥2 doses of naloxone.Citation24
A third study that also collected data in the 2016 timeframe assessed MNA among participants enrolled in the University of New Mexico’s Addiction and Substance Abuse Program (UNM ASAP).Citation25 Of the 251 study participants who completed 3 and 6 month follow-up interviews from April to October 2016, 44 participants performed 65 opioid overdose reversals. Of the 65 overdose events, ≥2 naloxone doses were utilized in 37 (57%) events during the 6-month follow-up period.
A fourth study by Somerville (2017) interviewed 64 individuals in Massachusetts in April 2016 who had witnessed a suspected IMF overdose in the past 5 months. This study found that 48 (75%) witnessed a suspected IMF overdose, and reported that ≥2 naloxone doses were administered in 40 (83%) of these overdose events.Citation26
More recently, three additional studies reported the proportion of MNA based on bystander self-report up to the 2020 timeframe. Mahonski assessed the rate of MNA among laypersons (including law enforcement officers [LEOs]) who were provided naloxone kits through an opioid overdose education and naloxone distribution (OEND) program in Maryland from 2015 to 2017.Citation27 Over 3 years, the proportion receiving ≥2 doses of naloxone rose from 16% (19/121) in 2015 to 20% (118/585) in 2017, representing a 25% increase. This corresponded with a significant increase in the mean naloxone dose from 2.12 mg (median: 2 mg) to 3.63 mg (median: 4 mg) during the same time frame (p < .0001). Notably, this study relied on individuals to report the overdose event to a regional poison control center on their own, which the authors noted were predominantly based on LEO-reported events. Thus the relatively low MNA rates may have been due to the lack of voluntary layperson reports.
A qualitative interview study that assessed the impact of 2 OEND training programs among individuals who use opioids found that between 2014 and 2019, 24 of 56 (43%) opioid overdose events included in the sample involved ≥2 naloxone administrations.Citation28
The most recent bystander self-report study in this review included 173 adults who used non-prescription opioids in the past 6 months between November 2019 and March 2020. This study reported that 54 of 61 (89%) patients received ≥2 doses of naloxone.Citation29 Of the participants who administered naloxone to someone else, 23 of 29 (79%) reported that more than 2 doses were needed to reverse the overdose. Notably, the most recent data collected from bystanders showed the highest rates of MNA, which may reflect the rising rates of IMF in the drug supply. Considering that the typical take home naloxone kit contains 2 doses of naloxone, these rising rates of MNA suggest the kits may need more naloxone to ensure an amount sufficient to reverse the overdose.
It is important to note that the generalizability of these bystander studies may be limited as each study had relatively small sample sizes, were conducted with participants within a defined geographic region and relied on bystander recall and self-report. However, despite these limitations, these 7 bystander studies offers further evidence of the increasing frequency of MNA.
Total naloxone doses administered
Two published studies of naloxone use for opioid overdose events reported the total naloxone dose, rather than number of doses. The first study was a single-center, retrospective chart review study that included both EMS and hospital EMR data from January 2017 to June 2018.Citation31 Total naloxone dose was compared between 3 study groups: IMF only (n = 23), opiates only (n = 28) and IMF + opiates (n = 70). Assessment of the total dose administered revealed that nearly 40% of the sample received a higher total naloxone dose over 0.4 mg. The median total dose did not differ significantly between the IMF only (0.80, 0.18–5.20) IMF + opiates (0.80, 0.18–3.60), and the opiates only (0.58, 0.18–2.00) involved group.Citation31 Limitations included the use of a qualitative, rather than quantitative urine immunoassay as a surrogate of opiate and IMF exposure by which the study groups were compared and the conversion of naloxone doses delivered via non-IV routes to an IV dose equivalent using literature-based assumptions, which may have lacked accuracy.
A study by Marco (2018) consisted of a questionnaire interview, supplemented by EMR data of adults presenting in the ED with an opioid overdose between July 2016 through July 2017.Citation32 Of the 89 participants, most (88%) received naloxone in the pre-hospital setting only. The mean prehospital naloxone dose administered was 5.8 mg (±5.0), and the mean total naloxone administered was 5.9 (±4.9).
Case series or studies
Four case series or case studies with sample sizes of ≤20 that reported MNA frequency were also included in this review. A retrospective public health investigation of an opioid overdose outbreak in West Virginia in August 2016 found that 6 of 20 (30%) events required ≥2 doses of naloxone. Of these, 5 were IV administrations of 0.4 mg per dose.Citation30 In a case series of 18 patients who presented with opioid toxicity at a single hospital in March 2016, 17 of 18 cases required ‘boluses of naloxone’ suggesting multiple doses were required for reversal.Citation33 A pilot study of 20 patients presenting to an urban tertiary care ED after a non-fatal opioid overdose found 50% received ≥2 doses of naloxone.Citation22 A final case study reported on a female presenting to the ED with severe opioid toxicity who required 17 mg total naloxone dose for reversal indicating MNA.Citation34
Discussion
Multiple naloxone administrations
Overall, this literature review found that the reported rates of MNA varied widely and have occurred in up to 89% of opioid overdose encounters in the US, were exceptionally high in certain regions, and have increased significantly over time. Among studies that utilized EMS data, MNA rates ranged from 9% to 53%,Citation16–22 while studies based on bystander-reported data were notably higher, ranging from 16% to 89%.Citation23–29 The wide range in reported MNA rates overall is not surprising given the significant variation in study design and attributes, the different data sources utilized, the year and geography studied, and the difficulty and limitations in accurately capturing this data. Most of the studies had relatively small sample sizes and were conducted in a limited geographic region, with only 2 studies based on national data with robust sample sizes. Thus, it is difficult to extrapolate an accurate rate of MNA for opioid overdose reversals in the US. However, given that lower MNA rates were generally reported in the EMS-based studies relative to the bystander-reported studies, there may be subtleties in the data sources or other factors that may explain differing MNA rates. For instance, lower MNA rates found in EMS-based studies may reflect variation in EMS protocols by state or EMS agency. Some states provide specific guidance on the maximum naloxone dose that should be administered, while other states recommend titrating naloxone until respiratory depression is resolved, but not necessarily to restore full consciousness.Citation9,Citation35,Citation36 Additionally, bystander interventions may not always be reported to EMS providers upon their arrival nor recorded by EMS, thus bystander administration might not be reliably recorded in EMS systems. EMS systems will also not include opioid overdose events that are treated and resolved solely by bystanders. Due to concerns about harassment or arrest, bystanders may not report prior naloxone administration to EMS or remain present when EMS arrives. For these reasons, EMS-based data may be reporting artificially lower MNA rates compared to other data sources that do not have these issues.
Association of IMF with multiple naloxone administrations
This review found very limited documentation on how rapidly IMF use in the real-world results in overdose. One study reported that in 36% of cases out of the 125 opioid overdose deaths, there was evidence an overdose occurring within seconds to minutes after drug use, and 90% were without a pulse upon EMS arrival.Citation26
This evidence of rapid IMF overdose onset, albeit limited, suggests that having a bystander who can administer enough doses of naloxone to reverse the overdose may be a critical factor in overdose survival. Given the increasing rates of bystander administration combined with the rising rates of IMF availability and the rapid onset of effect, bystanders should have training and access to enough naloxone to administer during the critical time interval before EMS services arrive to increase the likelihood of survival.
A recently developed opioid receptor quantitative systems pharmacology model simulated the effects of a single dose of 2 mg, 5 mg and 10 mg intramuscular (IM) naloxone dosage formulations on 3 different levels of fentanyl exposure (25 ng/ml, 50 ng/ml, 75 ng/ml).Citation37 When simulating the time predicted to reduce µ-receptor occupancy by fentanyl to below 50%, a trend emerged whereby increasing the naloxone dose resulted in faster time to achieve this threshold. The model predicted it would take 10 min to reduce the fentanyl receptor occupancy by 50% with a 2 mg IM (or 4 mg intranasal) naloxone dose based on 50 ng/ml level of fentanyl exposure, which the authors noted may be too long to successfully reverse the overdose. However, with higher naloxone dose administrations, the time to reduce fentanyl receptor occupancy to 50% was less than 5 min, suggesting that due to increased fentanyl exposure, higher doses of naloxone may be warranted.
Opioid withdrawal syndrome (OWS)
A concern with increasing naloxone dosing is the likelihood of an individual experiencing OWS precipitated by an abrupt reversal of the overdose. Only 5 studies in this review documented OWS or other adverse events related to naloxone and reported a range from 0.6% to 38%.Citation20,Citation21,Citation24,Citation27,Citation31 A recent systematic literature review evaluating naloxone dosing and adverse events (AEs) found that only half of the reviewed studies (86/174) reported AEs, and among these, 11% of patients experienced OWS.Citation38 The lack of documented cases of OWS following increased naloxone dosing reported could suggest that the risk is low, but it may also reflect the difficulty in capturing this metric, thus more research is needed to better understand the likelihood of OWS with increased naloxone dosing. However, given the life-threatening consequences of administering inadequate dosing for reversal, and the context of unknown IMF exposure, the risk of an unsuccessful reversal may outweigh the risk of OWS.
Other considerations
Finally, an unprecedented global health threat, the COVID-19 pandemic, has further exacerbated an already challenging opioid crisis. A recent study of a national sample of 872,762 urine specimens, which compared the period between the pre-COVID-19 baseline (January 1, 2019–March 14, 2020) to the COVID-19 pandemic period (March 15–May 16, 2020), reported a 35% (p < .01) significantly increased positivity rate for IMF during the COVID-19 period.Citation39 As the COVID-19 pandemic persists, it is expected that IMF will continue to rise which will act as a further catalyst to the pre-COVID-19 opioid overdose rate of progression.
Limitations
Several limitations to this review are noted. First, a systematic literature review (SLR) was not conducted due to the small number of published studies examining the frequency of MNA for opioid overdose reversals. All published studies that we could find that reported MNA for opioid overdose reversal were included in this review, regardless of study robustness or small sample sizes.
Another limitation was the paucity of large, national studies examining MNA. Most of the studies in this review had small samples sizes from relatively limited geographic areas, which may not be necessarily representative of the US. Two studies utilized the NEMSIS data, which is a large, national database of EMS encounters. It should be noted however that there are issues with representativeness even with the large NEMSIS database, as there is variation among states in the selection criteria used to submit EMS data to NEMSIS.Citation40 Furthermore, the two studies in this review that utilized this database did not include bystander administration of naloxone prior to EMS arrival in their calculation of MNA, thereby potentially underestimating the true rates of MNA.
Conclusion
This summary found that multiple naloxone administrations during opioid overdose encounters have increased over time and appeared to be exceptionally high in certain US regions. Lower rates of MNA reported in EMS-based studies relative to bystander-based studies may partially reflect variation in EMS guidelines by state and data limitations. Overall, evidence is emerging that higher formulations of naloxone may fulfill an unmet need, particularly given the increasing rates of IMF exposure. Further studies are needed to gain a better understanding of more recent occurrences of MNA during opioid overdose encounters, particularly across a wider geographic region in the US in order to inform continuing efforts to combat the opioid epidemic.
Authors’ contributions
Randa Abdelal, A. Raja Banerjee, Neyla Darwaza, Diane Ito and Josh Epstein were involved in the conception and design of this review, analysis, interpretation of the results and drafting/revising the paper for intellectual content. Suzanne Carlberg-Racich was involved in the interpretation of the results and drafting/revising the paper for intellectual content. All authors were involved in the final approval of this manuscript to be published and agree to be accountable for all aspects of the work herein.
Disclosure statement
Suzanne Carlberg-Racich has received consultancy fees from Hikma Community Health, a Subdivision of Hikma Specialty USA Inc. A. Raja Banerjee, Randa Abdelal, and Neyla Darwaza have received stock options exercisable for, and other stock awards of, ordinary shares of Hikma Pharmaceuticals in the course of their employment at Hikma Pharmaceuticals. Diane Ito and Josh Epstein are employees of Stratevi, a consulting firm that has received research funding from Hikma Community Health, a Subdivision of Hikma Specialty USA Inc., to conduct this review.
Additional information
Funding
References
- National Institute on Drug Abuse. Overdose death rates, 1999–2018. Available at: https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates. Accessed March 25, 2020.
- Substance Abuse and Mental Health Services Administration. 2019. Key substance use and mental health indicators in the United States: Results from the 2018 national survey on drug use and health. (HHS Publication No. PEP19-5068, NSDUH Series H-54). Rockville, MD: Center for Behavioral Health Statistics and Quality. Available at: https://www.samhsa.gov/data/.
- Comer SD, Cahill CM. Fentanyl: Receptor pharmacology, abuse potential, and implications for treatment. Neurosci Biobehav Rev. 2019;106:49–57.
- Centers for Disease Control and Prevention, National Center for Health Statistics. Multiple cause of death 1999-2015 on CDC WONDER online database. Released 2016.
- Gladden RM, Martinez P, Seth P. Fentanyl law enforcement submissions and increases in synthetic opioid–involved overdose deaths – 27 states, 2013–2014. MMWR Morb Mortal Wkly Rep. 2016;65(33):837–843.
- Wilson N, Kariisa M, Seth P, Smith H, Davis NL. Drug and opioid-involved overdose deaths – United States, 2017-2018. MMWR Morb Mortal Wkly Rep. 2020;69(11):290–297.
- Hedegaard H, Miniño AM, Warner M. Drug overdose deaths in the United States, 1999–2018. NCHS Data Brief, no 356. Hyattsville, MD: National Center for Health Statistics. 2020.
- O’Donnell J, Gladden RM, Mattson CL, Hunter CT, Davis NL. Vital signs: characteristics of drug overdose deaths involving opioids and stimulants – 24 states and the District of Columbia, January-June 2019. MMWR Morb Mortal Wkly Rep. 2020;59:1189–1197.
- North Carolina Chapter of Emergency Physicians. North Carolina Chapter of Emergency Physicians Treatment Protocol. 2020.
- Food and Drug Administration. Information about naloxone. Available at: https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/information-about-naloxone. Accessed March 08, 2021.
- Ryan SA, Dunne RB. Pharmacokinetic properties of intranasal and injectable formulations of naloxone for community use: a systematic review. Pain Manag. 2018;8(3):231–245.
- Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain – United States, 2016. MMWR Recomm Rep. 2016;65(1):1–50.
- American Medical Association. Opioids-lifesaving naloxone should be available almost anywhere. June 11, 2019. Available at: https://www.ama-assn.org/delivering-care/opioids/lifesaving-naloxone-should-be-available-almost-everywhere. Accessed October 22, 2020.
- Center for Disease Control and Prevention. Community-based opioid overdose prevention programs providing naloxone – United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61(6):100–105.
- Mattson CL, O'Donnell J, Kariisa M, Seth P, Scholl L, Gladden RM. Opportunities to prevent overdose deaths involving prescription and illicit opioids, 11 states, July 2016-June 2017. MMWR Morb Mortal Wkly Rep. 2018;67(34):945–951.
- Faul M, Lurie P, Kinsman JM, Dailey MW, Crabaugh C, Sasser SM. Multiple naloxone administrations among emergency medical service providers is increasing. Prehosp Emerg Care. 2017;21(4):411–419.
- Geiger C, Smart R, Stein BD. Who receives naloxone from emergency medical services? Characteristics of calls and recent trends. Subst Abus. 2020;41(3):400–407.
- Klebacher R, Harris MI, Ariyaprakai N, et al. Incidence of naloxone redosing in the age of the new opioid epidemic. Prehosp Emerg Care. 2017;21(6):682–687.
- Ga FP, Jr. Patel Carroll G, Bartimus H, Hunter K, Hong R. Do patients require emergency department interventions after prehospital naloxone? J Addict Med. 2020;14(3):224–230.
- Purssell R, Godwin J, Moe J, et al. 2020. Comparison of rates of opioid withdrawal symptoms and reversal of opioid toxicity in patients treated with two naloxone dosing regimens: a retrospective cohort study. Clin Toxicol.
- Maloney LM, Alptunaer T, Coleman G, et al. Prehospital naloxone and emergency department adverse events: a dose dependent relationship. Prehospital Care. 2020;59(6):872–883.
- Krotulski AJ, Chapman BP, Marks SJ, et al. 2021. Sentanyl: a comparison of blood fentanyl concentrations and naloxone dosing after non-fatal overdose. Clin Toxicol.
- Bell A, Bennett AS, Jones TS, Doe-Simkins M, Williams LD. Amount of naloxone used to reverse opioid overdoses outside of medical practice in a city with increasing illicitly manufactured fentanyl in illicit drug supply. Subst Abuse. 2019;40(1):52–55.
- Avetian GK, Fiuty P, Mazzella S, Koppa D, Heye V, Hebbar P. Use of naloxone nasal spray 4mg in the community setting: a survey of use by community organizations. Curr Med Res Opin. 2018;34(4):573–576.
- Katzman JG, Greenberg NH, Takeda MY, Balasch MM. Characteristics of patients with opioid use disorder associated with performing overdose reversals in the community: an opioid treatment program analysis. J Addict Med. 2019;13(2):131–138.
- Somerville NJ, O'Donnell J, Gladden RM, et. al. Characteristics of fentanyl overdose – Massachusetts, 2014-2016. MMWR Morb Mortal Wkly Rep. 2017;66(14):382–386.
- Mahonski SG, Leonard JB, Gatz D, Seung H, Haas EE, Kim HK. Prepacked naloxone administration for suspected opioid overdose in the era of illicitly manufactured fentanyl: a retrospective study of regional poison center data. Clin Toxicol.
- Parkin S, Neale J, Brown C, et al. 2021. A qualitative study of repeat naloxone administrations during opioid overdose intervention by people who use opioids in New York City. Int J Drug Policy.
- Schneider KE, Urquhart GJ, Rouhani S, et al. 2021. Practical implications of naloxone knowledge among suburban people who use opioids. Harm Reduct J.
- Massey J, Kilkenny M, Batdorf S, et al. Opioid overdose outbreak – West Virginia, August 2016. MMWR Morb Mortal Wkly Rep. 2017;66(37):975–980.
- Carpenter J, Murray BP, Atti S, Moran TP, Yancey A, Morgan B. Naloxone dosing after opioid overdose in the era of illicitly manufactured fentanyl. J Med Toxicol. 2020;16(1):41–48.
- Marco CA, Trautman W, Cook A, et al. Naloxone use among emergency department patients with opioid overdose. J Emerg Med. 2018;55(1):64–70.
- Sutter ME, Gerona RR, Davis T, et al. Fatal fentanyl: one pill can kill. Acad Emerg Med. 2017;24(1):106–113.
- Solomon R. A 20-year old woman with severe opioid toxicity. J Emerg Nurs. 2018;44(1):77–78.
- Rhode Island Department of Health. Rhode Island Statewide Emergency Medical Services Protocols, Version 2020. 01.
- New Hampshire Department of Safety. State of New Hampshire Patient Care Protocols, Version 8.0. 2018.
- Moss RB, McCabe Pryor M, Baillie R, et al. Higher naloxone dosing in a quantitative systems pharmacology model that predicts naloxone-fentanyl competition at the opioid mu receptor level. Plos One. 2020;15(6):e0234683.
- Moe J, Godwin J, Purssell R, et al. Naloxone dosing in the era of ultra-potent opioid overdoses: a systematic review. CJEM. 2020;22(2):178–186.
- Niles JK, Gudin J, Radcliff J, Kaufman HW. 2020. The opioid epidemic within the COVID-19 pandemic: drug testing in 2020. Population Health Management.
- National EMS Database – NEMSIS public release research data set V3.4.0. 2018 User manual. Office of Emergency Medical Services, National Highway Traffic Safety Administration. 2019.
- Clark AK, Wilder CM, Winstanley EL. A systematic review of community opioid overdose prevention and naloxone distribution programs. J Addict Med. 2014;8(3):153–163.
- Drug Enforcement Administration. National Drug Threat Assessment. Washington, DC: US Department of Justice; 2019.
- Vermont Department of Health. Vermont Statewide Emergency Services Protocol, 2000.