?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This study investigated activity, preferred pen location and social interactions in female piglets (0–10 weeks of age, N = 98) intended for breeding. Piglets were housed in pens where the sow and the piglets were loose-housed without (CP) or with access to the neighbouring pen week 2–5 (AP). Female piglets of two genetic lines (Dutch and Swedish Yorkshire (DY, SY)) from 26 litters were selected within 24 h after birth. DY piglets in the AP treatment spent more time in the neighbouring pen than SY (24.0% vs 19.0%), while AP piglets of both genetic lines spent less time lying down before weaning than CP. At weaning, CP piglets increased their time in the piglet corner and spent less time lying. SY piglets were less responsive to social interactions. The results confirm previous findings on favourable effects of early social mixing on piglets’ behavioural responses to weaning also when sows are individually loose-housed.
Introduction
As pigs are social animals, European Union (EU) regulations require group housing of pigs, with the exception of sows around farrowing and adult boars. However, mixing unfamiliar pigs often results in aggression, which can lead to injury, suffering and social stress, with negative effects on health and productivity (e.g. Arey & Edwards, Citation1998; Greenwood et al., Citation2014; Peden et al., Citation2018).
Piglets start to form relationships and establish a social hierarchy within the litter only a few hours after birth (Graves, Citation1984). Under feral and free-range conditions, piglets are involved in social interactions with unfamiliar piglets from other litters and older conspecifics of different ages in the maternal group of their mother from the first 1–2 weeks after birth (Jensen, Citation1986; Petersen et al., Citation1989; Wechsler, Citation1996). In modern pig production, the first mixing of unfamiliar pigs commonly occurs much later, after weaning, which usually occurs at around four to 5 weeks of age. After birth and survival during the first week in life, weaning is the next major challenge for piglets in modern pig production, with long-term effects on pig welfare and production, illustrated by stress responses altering behaviour, impaired performance including growth lag and gastrointestinal tract disorders leading to diarrhoea (e.g. Blavi et al., Citation2021; Van Kerschaver et al., Citation2023).
With the change in EU regulations (EU Directive on minimum standards for the protection of pigs (2008/120/EC)) requiring group housing instead of individual stalls for sows during gestation, there has been an increased focus among pig producers, authorities and researchers on management of group-housed sows, including prevention of negative effects such as injurious aggressive behaviour (Greenwood et al., Citation2014; Peden et al., Citation2018). Methods to reduce such unwanted damaging behaviours in commercial pig production have been sought, initially focusing on space allowance and group size, but in later years also on genetic selection, nutritional aspects, early-life socialisation (also termed co-mingling) and use of pheromones (e.g. Greenwood et al., Citation2014; Peden et al., Citation2018; Ko et al., Citation2020; Rydhmer, Citation2020). Early-life socialisation is reported to have long-term benefits such as fewer aggressive interactions when meeting unfamiliar pigs later in life (e.g. Salazar et al., Citation2018; Ko et al., Citation2020). Methods shown to reduce unwanted damaging behaviours in group-housed adult sows are likely also to affect the sows early in life, including the period pre- and post-weaning (Van Kerschaver et al., Citation2023). For example, a combination of pre-weaning socialisation and environmental enrichment in crated farrowing and nursing pens has been shown to reduce stress relating to weaning and post-weaning aggression in pigs intended for slaughter (Ko et al., Citation2020). However, the majority of the studies reported on mixing and co-mingling of piglets during the nursing period have investigated systems where several sows and their piglets are housed together in groups or systems with individual sow housing where sows are crated. Reports on the effects of mixing and co-mingling of piglets in systems where sows are individually loose-housed during the nursing period are scarce (Van Kerschaver et al., Citation2023).
Genetic selection can influence behaviour, e.g. through breeding for less aggressive animals (Løvendahl et al., Citation2005; Turner et al., Citation2010; Peden et al., Citation2018; Rydhmer, Citation2020). Current breeding goals often focus on the same traits in most pig dam lines (i.e. improved litter size, piglet growth, piglet survival, sow and piglet health, etc.), but the production environment in which the animals are evaluated and selected may play an important role through indirect selection of traits not included in breeding goals, e.g. behaviours beneficial for coping in the specific production environment. Under the Swedish Animal Welfare Act 1988 (SFS 1988:534), individual stalls for sows during insemination and pregnancy and crated housing for sows during farrowing and nursing have long been banned in Sweden, unlike in many other countries (Einarsson et al., Citation2014). Therefore sows in Swedish pig production have been group-housed during insemination and pregnancy, and housed in individual loose-housing pens with their litters during farrowing and nursing, for several decades. Moreover, according to the Swedish Animal Welfare Act 1988 and the current version (SFS 2018:1192), all pigs must have access to straw at all times. Thus the Swedish Yorkshire genetic line selected for Sweden’s rather unique production environment for sows (from an international point of view) may have been indirectly selected for behaviours beneficial for group housing of dry sows and loose-housed nursing. In parallel, sow lines such as Dutch Yorkshire (DY) may have been indirectly selected for behaviours beneficial in individual stalls (Horback & Parsons, Citation2016).
The objective of this study was to determine the effects of genetic line (breeding) and early-life social mixing (co-mingling) on female piglet activity, preferred pen location and social interactions pre- and post-weaning in a housing system with individually loose-housed sows. The hypotheses were that: (i) female piglets reared with access to unfamiliar piglets are more active and better prepared for the challenges related to weaning, as indicated by more initiation of social interactions and less time spent inactive in the piglet corner after weaning; and (ii) piglet activity, preferred pen location and social interactions differ between genetic lines evaluated and selected in different social environments.
Materials and methods
The experimental work was performed at the Swedish Livestock Research Centre, Lövsta, Uppsala, Sweden, during January 2018-January 2019. The experiment and all procedures involved were approved by the National Ethics Committee for Animal Experiments in Uppsala (Registration number: 5.8.18-16279/2017).
Animals, housing, and management
A total of 98 female piglets from two genetic dam lines, Dutch Yorkshire (DY; Topigs Norsvin distributed through Svenska Köttföretagen) and Swedish Yorkshire (SY; Nordic Genetics) (Lundeheim, Citation2017) were used. Only female piglets destined for gilt recruitment were included in the study, as the methods tested are intended to reduce damaging behaviours related to group housing in adult sows. The distribution of the 98 female piglets between genetic lines and early social mixing environments is shown in .
Table 1. Number of female piglets (number of litters) of the genetic lines Dutch Yorkshire (DY) and Swedish Yorkshire (SY) allocated to two early social mixing environments, one where piglets had access to the sow and piglets in a neighbouring pen (AP) and one where piglets were confined to their own pen (CP) during the early socialisation phase (2–5 weeks of age).
The female piglets were studied from birth until 10 weeks of age and originated from 26 litters divided over seven farrowing batches (A-G). In each farrowing batch, two pens were allocated to an access pen treatment (AP) and two pens to a control closed pen treatment (CP). The sow of each litter was moved into a loose-house farrowing and nursing pen approximately one week before expected farrowing. There were no gates for confining sows in the pen and thus all sows were loose-housed at all times, including during and in the first days after farrowing. Multiparous sows of each genetic line were allocated randomly to either an AP or CP pen. The sow stayed in the pen until weaning of her piglets, at approximately five weeks (34.3 ± 1.86 days) after birth. The piglets stayed in the pen until approximately 10 weeks of age (69.2 ± 1.70 days).
Within 24 h after birth, four female piglets in each litter were selected for detailed observation. If there were more than four female piglets in the litter, four were randomly selected (but excluding the heaviest and/or the lightest). There were fewer than four female piglets in five of the 26 litters and in those cases only the female piglets available (2, 3, 3, 3, and 3 female piglets, respectively) were observed. For very large and very small litters, cross-fostering (within genetic line) was applied within two days after birth. However, none of the female piglets selected for the study was cross-fostered.
The pens measured 3.35 m × 2.00 m in total and were divided into three sections: a concrete-floored lying and feeding area (2.10 m × 2.00 m), a slatted dunging area (1.25 m × 2.00 m) and a piglet corner accessible only to the piglets with floor heating, a roof and a headlamp (). The floor heating was turned on from before farrowing until one week after weaning. The heat lamp and the roof was always in place until week 3 after farrowing, and was taken away between weeks 3 and 5 after weaning depending primarily on season (i.e. climate differences between winter and summer) and piglet behaviour.
Each sow was provided with 15–20 kg chopped straw two days prior to the calculated date of farrowing. This straw was gradually lost through the slatted floor and an additional 0.5–1 kg straw was then provided, in accordance with common Swedish management routines to ensure that straw was always available for the sows. The pens were cleaned manually every morning. The sows were initially fed a standard commercial dry feed for lactating sows two times per day, via an automatic feeding system. When the piglets reached approximately 10 days of age, the feeding regime was extended to include one extra feed, i.e. the sows were then fed three times a day until the piglets were weaned. Dry feed adapted for piglets was provided on the floor, at a rate of 200 g per day, from when the piglets were approximately two weeks old, and an ad libitum feed dispenser was added in the piglet corner when the piglets reached approximately three weeks of age. Water was available ad libitum from two drinking nipples, placed one over the other, at 100 and 150 mm above the slatted floor ().
All piglets received a 1 mL intramuscular injection of iron supplement (Uniferon, 200 mg/mL) and were ear-tagged at approximately four days of age (3.8 ± 0.78 days). The female piglets selected for observation were marked with ear-tags of individual colours other than yellow (all other piglets in the litter had yellow ear-tags) in preparation for behaviour observations. A second injection with the same amount of iron was administered at approximately two weeks of age (13.1 ± 1.79 days).
The health of the pigs was monitored continuously by farm staff and any issues arising were treated and documented. Piglets were weighed at birth and five weeks of age.
Early social mixing
The selected female piglets and their siblings were allocated to one of two different social housing environments. In two of the four pens in each farrowing unit, a pop-hole was made in the piglet corner connected to the neighbouring pen (). This allowed piglets, but not sows, to move between the two pens, thus creating an extended social environment for the piglets in the AP treatment. The other two pens in each farrowing unit were conventional loose-house closed farrowing pens (CP), used as a control. Apart from the pop-hole, there were no differences between AP and CP housing. The early social mixing environment examined (between weeks 2 and 5) was chosen because it corresponds to the time when piglets in the wild meet new piglets (Jensen, Citation1986).
When the piglets reached approximately two weeks of age (13.1 ± 1.79 days; DY: 13.2 ± 1.65 days, SY: 13.0 ± 1.96 days), the pop-hole in the AP pens was opened. In the experimental design, balancing genetic line and early social mixing environment, four sows and their litters were included in each farrowing batch, two sows from each genetic line. One sow and her litter from each genetic line were then randomly allocated to each of the early social environment treatments. The intention was to have one CP per genetic line and one AP per genetic line for each batch in neighbouring pens, meaning that litters mixing in the AP treatment would be of different genetic lines. This was the case for 11 of the 13 litters (84.6%) in the AP treatment. The two AP treatment litters that did not meet the opposite genetic line met other litters not included in the study (one DY litter met crossbred SY*DY piglets in the neighbouring pen, while the other DY litter met another DY litter). The pop-hole was left open until weaning at five weeks of age (34.3 ± 1.86 days), after which the piglets were kept in their original pens and could not access the neighbouring pen again.
Behaviour and pen location observations
Each individual female piglet was treated as an observation unit and was observed on eight occasions during the study period ().
Figure 2. Timing of behaviour observations and early social mixings (access to neighbouring pen (AP) or closed pen (CP)). *The observation in week 2 was made on the day before opening the pop-hole, while the observation in week 5 was made on the day after the pop-hole was closed and the sow was moved from the pen (at weaning).

Protocols for observation of body posture, preferred location in pen and social behaviour () were developed in a pilot study (Vahlberg, Citation2019). For AP piglets, a distinction was made during the observations in weeks 3 and 4 on whether the piglet was in its home pen or the neighbouring pen when observations were made on body posture and location in pen. A similar distinction was not made for the AP piglets in weeks 2 and 5, as observations in week 2 were made on the day before opening the pop-hole and those in week 5 were made on the day after the pop-hole was closed and after the sow had been moved from the pen ().
Table 2. Ethogram of behaviours recorded with scan and continuous sampling.
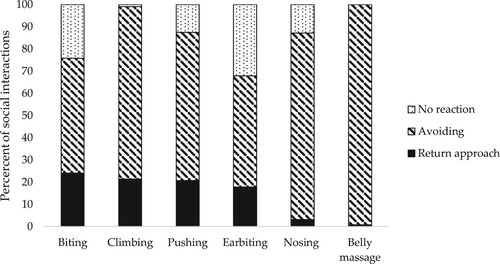
Social interactions were recorded in continuous observations, while body position and position in the pen were recorded with scan sampling. All behaviour observations were performed directly from outside the pen between 08:00 and 16:00 h, after the piglets were habituated to the observer (8 min). The observations started with scan sampling of all female piglets from each farrowing batch in each of the four pens, followed by continuous one-minute observations of two female piglets from each home pen. This routine was repeated until all female piglets had been scanned 17 times and continuously observed twice (i.e. two minutes of continuous observation per occasion), giving a total of 136 scan samples and 16 min of continuous observation per female piglet for the eight observation occasions. If female piglets in AP pens were in the neighbouring pen during the observation, both pens were observed simultaneously. The nature of a social interaction is not defined solely by the behaviour of the performing pig, and must also consider the reaction of the receiving pig (McGlone, Citation1985; Newberry et al., Citation1988). Thus in addition to analyses of social behaviours, the reaction/behaviour of the receiving piglet was also recorded (as return approach, avoiding or no reaction) ().
The observations were made by two observers, with one main observer making 72.8% of the observations. To determine inter-observer reliability, both observers made simultaneous observations on six different occasions (112 direct observation minutes and 432 scans) and the degree of agreement was assessed using the kappa method in procedure FREQ in SAS. The agreement between the two observers was strong, with kappa values >0.95.
Statistical analyses
Statistical analyses were performed using SAS software (version 9.4 of the SAS system for Windows© 2016, SAS Institute Inc., Cary, NC, USA). The statistical unit assessed was animal (taking the litter into account) per observation occasion (week). Scan-recorded piglet activity and pen location were converted to percentage of scans per animal and observation occasion. Before the statistical analyses, continuously observed social behaviour variables, i.e. performing and receiving social behaviour, were transformed into binomial variables (female piglets performing each initiating social behaviour (or not) (), and female piglets responding to a social interaction with return approach (or not), avoiding (or not), or no reaction (or not) in each observation week).
Residuals of the continuous (not binomially distributed) dependent variables were examined for normal distribution using PROC UNIVARIATE considering Shapiro-Wilk’s test and normal probability plots. All residual variables tested were found to be normally or approximately normally distributed. Results are presented as least squares means (LSM) with standard error (±SE), unless otherwise stated.
Statistical models were developed using step-backward selection of predictor effects where non-significant interactions and effects were deleted from the model. Statistical models were developed separately for general linear mixed models (normally distributed variables) and generalised mixed linear models (binomially distributed variables). Differences between genetic lines (DY and SY), early social mixing (AP and CP), and over time (1, 2, 3, 4, 5, 6, 9, 10 weeks of age) were analysed with PROC MIXED for continuous variables recorded with scan sampling (percentage of time lying and percentage of time in the piglet corner). Binomial variables recorded with continuous sampling (observations where female piglets initiated social interactions with lifting, pushing, climbing, mounting, biting on body, tail biting, vulva biting, ear biting, head knock and two additional variables, one merging all types of biting behaviour (Biting total) and one merging initiating any type of social behaviours (Performing Social interaction total), and observations where female piglets responded to a social interaction with return approach, avoiding, or no reaction) were analysed by PROC GLIMMIX, using binomial distribution and a logit link function. The following model was used for both continuous and binomial variables:
where y is the analysed variable, with genetic line (DY or SY), early social mixing (AP or CP), batch (A, B, C, D, E, F, G), observation week (1, 2, 3, 4, 5, 6, 9, 10), the interaction between genetic line and observation week, and the interaction between early social mixing and observation week included as fixed effects, and animal (N = 98 nested within genetic line, early social mixing, and batch) included as a repeated random effect; and e is random error effect. Due to the design of the study, the effect of animal nested within genetic line, early social mixing, and batch included the effect of birth litter.
The interaction between genetic line and early social mixing was tested during development of the statistical model but was found not to be significant (p > 0.05), so it was not included in the final model.
Differences between the genetic lines and between early social mixing treatments in piglet weight at birth, weaning (5 weeks) and 10 weeks of age were analysed with piglet as the statistical unit and with general linear mixed models using PROC MIXED with the following model:
where y is the analysed variable, with genetic line (DY or SY), early social mixing (AP or CP), batch (A, B, C, D, E, F, G) included as fixed effects, and litter (N = 26 nested within genetic line, early social mixing, and batch) included as a random effect; and e is random error effect.
Differences in litter size and piglet mortality between genetic lines and between early social mixing treatments were analysed with litter as the statistical unit. As no significant interactions between genetic line and early social mixing were found, pairwise analyses of genetic lines and of early social mixing environments were performed with general linear models using PROC GLM with the following model:
where y is the analysed variable, with genetic line (DY or SY), early social mixing (AP or CP), batch (A, B, C, D, E, F and G) and e as random error effect.
Differences in disease prevalence between genetic lines and between early social mixing treatments, differences between observation weeks and genetic lines in percentage of female piglets in neighbouring pens in the AP pens, and differences in percentage of observations in different pen locations in the neighbouring pen and body positions between genetic lines in the AP pens in weeks 3 and 4 were analysed with Chi square tests using PROC FREQ.
Results
There was no difference in litter size at birth between litters born in AP and CP, but by 5 and 10 weeks of age litter size was significantly larger in AP, due to numerically higher piglet mortality in CP litters (although the difference was not significant due to large variation between litters). Average litter size was greater for DY sows compared with SY sows ().
Table 3. Litter size, piglet mortality and mean piglet weight for Dutch Yorkshire (DY) and Swedish Yorkshire (SY) litters, and for litters in access pens (AP) and closed pens (CP) (LSM ± SE).
The primary reason for medical treatment among the female piglets was leg problems, including joint inflammation, leg injuries and hoof damage. Of the DY piglets, 14.8% were treated for leg problems, compared with 4.6% of the SY piglets (p = 0.095). For female piglets housed in AP and CP, the percentage treated for leg problems was 10.2% in both treatments. Of the DY piglets, 13.5% were treated for illnesses other than leg problems, such as wounds or infections, compared with 2.4% of the SY piglets (p = 0.056). The corresponding value for AP piglets was 4.1%, compared with 12.2% for CP piglets CP (p = 0.140).
Location
Female piglets in AP pens were observed in the neighbouring pen for 24.1% of the time during week 3 observations, compared with 20.0% of the time in week 4 (p = 0.045). Of the female piglets in AP, SY piglets were located in the neighbouring pen for a smaller proportion of time (scans) than DY piglets (19.0% and 24.0%, respectively; p = 0.017). All female piglets in the AP pens were observed in the neighbouring pen at least once. In observation scans in weeks 3 and 4, AP piglets observed in the neighbouring pen spent 55.4% of the time lying, with no significant differences between the genetic lines. However, presence in the piglet corner of the neighbouring pen differed between genetic lines, in that DY female piglets spent 58.0% of the observed scans in the neighbouring pen located in the piglet corner, compared with 72.4% for SY female piglets (p = 0.007).
The female piglets observed in the present study spent the majority of their time located either in the lying area with the sow or in the piglet corner (). For time spent in the piglet corner, there were significant interactions between early social mixing and observation week (p = 0.001) (), and between genetic line and observation week (p = 0.005) (). There was no clear pattern in the differences between early social mixing or between genetic lines, but there were differences between weeks in how much time the female piglets spent in the piglet corner. For example, there was a large increase in time spent in the piglet corner directly after weaning, especially for female piglets housed in CP ( and ). Prior to weaning, female piglets in the CP pens had been decreasing their time in the piglet corner. There was a corresponding increase in time spent in other areas of the pen, i.e. if the piglets were not in the piglet corner they were in the lying or slatted area.
Figure 3. Percentage of scans (time) spent in the piglet corner by female piglets housed in access pens (AP) and closed pens (CP) in observation weeks 1–10. LSM ± SE. In total 17 scans per female piglet and observation week. For observations in weeks 3 and 4 in AP pens, when the piglets had access to both the home and neighbouring pen, observations in the piglet corner in both pens are included. Weaning occurred at week 5. N = 784 scans. Significance levels for pairwise differences within observation week are indicated: ***p < 0.001, *0.01 < p < 0.05.
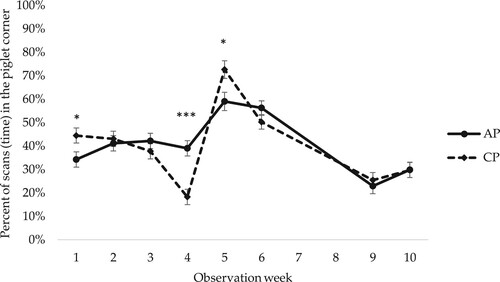
Figure 4. Percentage of scans (time) spent in the piglet corner by Swedish Yorkshire (SY) and Dutch Yorkshire (DY) female piglets in observation weeks 1–10. In total 17 scans per female piglet and observation week. LSM ± SE. For observations in weeks 3 and 4 in AP pens, when the piglets had access to both the home and neighbouring pen, observations in the piglet corner in both pens are included. Weaning occurred at week 5. N = 784 scans. Significance levels for pairwise differences within observation week are indicated: *0.01 < p < 0.05, †0.05 < p < 0.1.
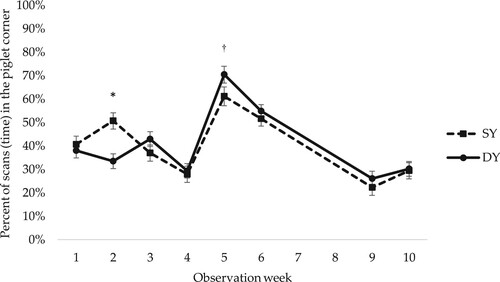
Table 4. Descriptive statistics on percentage of scans (time) (in total 17 scans per female piglet and observation week, mean % ± standard deviation) spent by Dutch Yorkshire (DY) and Swedish Yorkshire (SY) female piglets, and by female piglets in access pens (AP) and control pens (CP), in different locations in the pen and in different body positions.
Body position
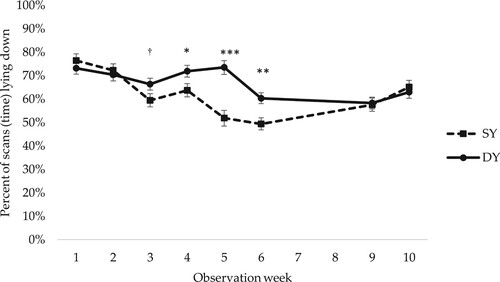
All female piglets spent the majority of their time lying down (). For percentage of time lying, there was a significant interaction between early social mixing and observation week (p = 0.001). Pairwise comparisons within observation week indicated that female piglets housed in CP spent a larger percentage of their time lying in the last few weeks before weaning (which occurred at five weeks of age) and then showed a large decrease in time spent lying after weaning, whereas female piglets in AP did not alter their lying behaviour after weaning (). There was also a significant interaction between observation week and genetic line (p = 0.001). Pairwise comarisons within observation week showed that DY female piglets spent a larger percentage of their time lying down from week 3 to week 6 after birth ().
Figure 5. Percentage of scans (time) spent lying by female piglets housed in access pens (AP) and closed pens (CP) in observation weeks 1–10. In total 17 scans per female piglet and observation week. LSM ± SE. For observations in weeks 3 and 4 in AP pens, when the piglets had access to both the home and neighbouring pen, observations in both pens are included. Weaning occurred at week 5. N = 784 pig observation scans. Significance levels for pairwise differences within observation week are indicated: ***p < 0.001, **0.001 < p < 0.01, *0.01 < p < 0.05.
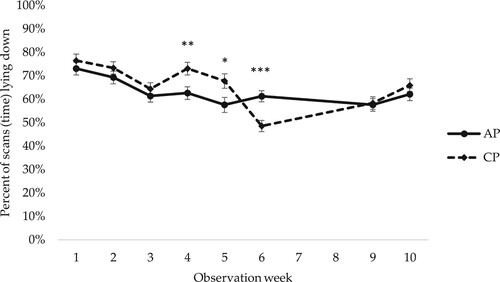
Figure 6. Percentage of scans (time) spent lying by Swedish Yorkshire (SY) and Dutch Yorkshire (DY) female piglets in observation weeks 1–10. In total 17 scans per female piglet and observation week. LSM ± SE. For observations in weeks 3 and 4 in AP pens, when the piglets had access to both the home and the neighbouring pen, observations in both pens are included. Weaning occurred at week 5. N = 784 scans. Significance levels for pairwise differences within observation week are indicated: ***p < 0.001, **0.001 < p < 0.01, *0.01 < p < 0.05, †0.05 < p < 0.1.
Social interactions
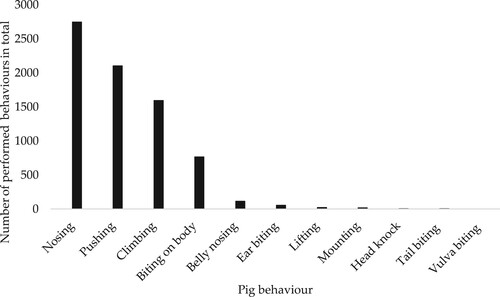
The most frequent social interaction initiated was nosing another pig, followed by interactions where the performing pig pushed or climbing on the receiving pig (). The proportion of social interactions per initiating behaviour that were met with the response ‘no reaction’, ‘avoiding or ‘return approach’ is presented in . SY female piglets showed ‘no reaction’ in response to a social interaction in a larger percentage of observations (88.2 ± 2.00%) than DY female piglets (78.9 ± 2.41%) (p = 0.005). There were no other significant effects of genetic line or early social mixing for any of the other performed or received social interactions analysed. There were no differences in the percentage of female piglets performing some kind of social interaction (‘performed social interactions total’) between the observation weeks, but the percentage of female piglets performing nosing at least once during the weekly observations increased gradually over time, from 31% in week 1 to 87% in week 10, for both DY and SY piglets and in both the AP and CP treatments (p = 0.001). Moreover, the percentage of female piglets performing biting (biting total) at least once during the weekly observations also increased gradually over time, from 8% in week 1 to 49% in week 10 (p = 0.001). In contrast, the percentage of female piglets initiating social interactions with climbing at least once during the weekly observations decreased gradually over time, from 61% in week 1 to 14% in week 10 (p = 0.001).
Discussion
The effects of early mixing and genetic line on female piglet activity, preferred pen location and social interactions pre- and post-weaning were compared under housing conditions feasible for implementing commercial pig production in Sweden. The key findings were effects of early mixing on changes in the behaviour of female piglets around weaning (greater behaviour change in CP piglets compared with AP piglets) and of genetic line on socialisation in the neighbouring pen (DY piglets spent a larger percentage of time in the neighbouring pen).
Weaning has frequently been reported to be challenging for piglets, causing e.g. deterioration in health and behaviour changes that have been linked to potential welfare problems and stress (Campbell et al., Citation2013; Matthews et al., Citation2016; Ko et al., Citation2020; Blavi et al., Citation2021; Van Kerschaver et al., Citation2023). In this study, female piglets housed in CP altered their time spent in the piglet corner and their lying behaviour after the sow had been moved from the pen to a larger extent than female piglets housed in AP. The increased time spent in the piglet corner after weaning seen in all piglets was probably caused by a combination of factors, such as seeking heat and social support from litter mates, but it was particularly noticeable in the CP treatment because the time in the piglet corner had been decreasing before weaning. Female piglets in the CP treatment decreased their time lying in the week after weaning, while the percentage of time lying remained stable around weaning for female piglets in the AP treatment. In combination, the altered lying behaviour observed for CP piglets and their greater use of the piglet corner may indicate that piglets in that treatment were more affected by weaning than piglets in AP. Potential reasons for this could be that AP pigs did not have the same attraction to the piglet corner in their home pen, as indicated by the finding that the AP piglets spent the majority of the weeks 3 and 4 scans in the piglet corner of the neighbouring pen, or that they were more accustomed to being away from their own mother and thus weaning was not as novel for them as for CP piglets. These results are in agreement with previous findings on differences in social behaviour and activity between socialised and unsocialised piglets (e.g. Kutzer et al., Citation2009; Salazar et al., Citation2018; Ko et al., Citation2020; Van Kerschaver et al., Citation2023). The findings of the present study support previous findings and shows that socialisation in piglets during nursing is beneficial for piglets handling challenges related to weaning also in commercial production environments with individually loose-housed sows and straw enrichment.
The female piglets in this study spent most of their time lying down, as also observed in several other studies (e.g. Hessel et al., Citation2006; Schrey et al., Citation2019). Regarding effects of early mixing on lying behaviour before weaning, the lower percentage of time lying among AP piglets in week 4 implied a higher level of activity that could have been due to the greater number of piglets available to play and be active with, and the larger space allowance created by the pop-hole (Chaloupková et al., Citation2007; Oostindjer et al., Citation2011; Singh et al., Citation2017; Salazar et al., Citation2018). This is partly supported by findings in previous studies where socialised piglets displayed a higher level of play behaviour from 14 days of age, hence displaying more active behaviour than non-socialised piglets (e.g. Salazar et al., Citation2018).
Regarding effects of genetic line on lying behaviour, DY female piglets spent more time lying down 3–6 weeks after birth than SY female piglets. A typical behaviour in ill pigs is reduced movement (Wilson et al., Citation2014). Thus one contributing explanation for the higher percentage of time spent lying by DY female piglets could be poorer health, as a higher percentage of those piglets were medically treated for leg problems or other illnesses than SY female piglets. There is no clear explanation for the poorer health in DY female piglets, but higher genetic potential for growth resulting in higher general sensitivity is probably part of the explanation.
An important element of the development of social behaviours in pigs is introduction of the piglet to unfamiliar sows and their piglets, corresponding to the reunion of sows and their piglets with the maternal social group that typically occurs at around 1–2 weeks after birth in wild and feral pigs (Jensen, Citation1986; Petersen et al., Citation1989; Wechsler, Citation1996). In the present study, female piglets in AP were frequently observed in the neighbouring pen, interacting with the piglets and sow in the other pen, as also seen in previous studies (Jensen & Redbo, Citation1987; Kutzer et al., Citation2009). Approximately 20% of AP female piglets were found in the neighbouring pen on the observation occasions when they had access to that pen. The percentage of time spent in the neighbouring pen differed between genetic lines, and was higher for DY compared with SY female piglets. This difference could be partly explained by the larger birth litters of DY compared with SY sows, since greater litter size increases piglet competition (Andersen et al., Citation2011; Kobek-Kjeldager et al., Citation2020). It is possible that DY female piglets took the opportunity to cross-suckle the sow in the neighbouring pen more often due to high competition at the udder of their own birth sow. This is supported by the high incidence and significantly higher proportion of cross-suckling in SY litters (i.e. DY piglets cross-suckled SY sows) found in a parallel pilot study assessing cross-suckling in the litters included in the present study (Lundahl, Citation2019). It is also supported by the finding in this study that during the observation scans where the AP female piglets were observed in the neighbouring pen in weeks 3 and 4, DY female piglets spent a lower percentage of scans in the piglet corner, and thus a higher percentage of the scan in pen locations where they could access the sow in the neighbouring pen.
In contrast to previous findings (e.g. Van Putten & Bure, Citation1997; Wattanakul et al., Citation1997; D’Eath, Citation2005) there were no differences in performance of social behaviours between genetic lines or social mixing environments investigated in this study. However, mapping of social behaviour over time indicated changes in social behaviour in the piglets with age. The percentage of female piglets performing nosing and biting increased over time, while the performance of climbing decreased, indicating gradual development of social behaviour during the five-week study period.
SY female piglets more often made no response to a social interaction than DY female piglets. Possible reasons are that SY female piglets were approached in social interactions to a lesser extent, that SY female piglets did not notice the social invitation, or that the threshold to respond to a social interaction was higher among SY than CP piglets. However, it is also likely that the smaller litter size in SY compared with DY litters contributed to the higher percentage of ‘no response’ reactions in SY piglets, as smaller litter size leads to less competition at the udder and fewer severe or agonistic social interactions between piglets. The difference could also be a result of the poorer health in DY compared with SY piglets, which may have caused more frequent agonistic social interactions among DY piglets due to e.g. pain and discomfort. However, since there were no other differences in social interactions performed or received between the genetic lines, the results imply that there are no major differences in social behaviour between female piglets of the two genetic lines.
Conclusions
This study revealed effects of early mixing on behaviour changes in female piglets around weaning and genetic line effects on socialisation with piglets in the neighbouring pen. Piglets housed in AP showed fewer signs of being affected by weaning than piglets housed in CP, as evidenced by smaller changes in behaviour from the week before, during and after weaning (weeks 2, 5 and 6, respectively) in AP piglets compared with CP piglets. Female piglets of the DY genetic line were more responsive to social interactions than female piglets of the SY line. The findings of this study support previous findings on favourable effects of early social mixing on piglets’ behavioural response to weaning and confirm the findings for commercial production environments with individually loose-housed sows and straw enrichment. Moreover, this study indicates limited effects of genetic line on piglet response to weaning.
Author contributions
L.M. Backeman Hannius: Conceptualisation, Investigation, Data curation, Formal analysis, Methodology, Project administration, Visualisation, Writing – original draft, Writing – review & following. L. Keeling: Conceptualisation, Funding acquisition, Methodology, Supervision, Writing – review & following. P. Ask-Gullstrand: Investigation, Methodology, Project administration, Writing – review & following. E. Verbeek: Methodology, Formal analysis, Supervision, Writing – review & following. A. Wallenbeck: Conceptualisation, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Visualisation, Writing – review & following. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
We would like to thank the farm staff at the Swedish Livestock Research Centre Lövsta (pig facility) for their support in conducting the experiment.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Andersen, I. L., Nævdal, E. & Bøe, K. E. (2011). Maternal investment, sibling competition, and offspring survival with increasing litter size and parity in pigs (Sus scrofa). Behavioral Ecology and Sociobiology, 65, 1159–1167. doi:10.1007/s00265-010-1128-4.
- Arey, D. S. & Edwards, S. A. (1998). Factors influencing aggression between sows after mixing and the consequences for welfare and production. Livestock Production Science, 56, 61–70.
- Blavi, L., Solà-Oriol, D., Llonch, P., López-Vergé, S., Martín-Orúe, S. M. & Pérez, J. F. (2021). Management and feeding strategies in early life to increase piglet performance and welfare around weaning: a review. Animals, 11, 302. doi:10.3390/ani11020302.
- Campbell, J. M., Crenshaw, J. D. & Polo, J. (2013). The biological stress of early weaned piglets. Journal of Animal Science and Biotechnology, 4, 1–4. doi:10.1186/2049-1891-4-19.
- Chaloupková, H., Illmann, G., Bartoš, L. & Špinka, M. (2007). The effect of pre-weaning housing on the play and agonistic behaviour of domestic pigs. Applied Animal Behaviour Science, 103, 25–34. doi:10.1016/j.applanim.2006.04.020.
- D’Eath, R. B. (2005). Socialising piglets before weaning improves social hierarchy formation when pigs are mixed post-weaning. Applied Animal Behaviour Science, 93, 199–211. doi:10.1016/j.applanim.2004.11.019.
- Einarsson, S., Sjunnesson, Y., Hultén, F., Eliasson-Selling, L., Dalin, A. M., Lundeheim, N. & Magnusson, U. (2014). A 25 years experience of group-housed sows-reproduction in animal welfare-friendly systems. Acta Veterinaria Scandinavica, 56, 37–45. doi:10.1186/1751-0147-56-37.
- Graves, H. B. (1984). Behavior and ecology of wild and feral swine (Sus scrofa). Journal of Animal Science, 58, 482–492. doi:10.2527/jas1984.582482x.
- Greenwood, E. C., Plush, K. J., van Wettere, W. H. E. J. & Hughes, P. E. (2014). Hierarchy formation in newly mixed, group housed sows and management strategies aimed at reducing its impact. Applied Animal Behaviour Science, 160, 1–11. doi:10.1016/j.applanim.2014.09.011.
- Hessel, E. F., Reiners, K. & Van Den Weghe, H. F. A. (2006). Socializing piglets before weaning: effects on behavior of lactating sows, pre- and postweaning behavior, and performance of piglets. Journal of Animal Science, 84, 2847–2855. doi:10.2527/jas.2005-606.
- Horback, K. M. & Parsons, T. D. (2016). Temporal stability of personality traits in group-housed gestating sows. Animal, 10, 1351–1359. doi:10.1017/S1751731116000215.
- Jensen, P. (1986). Observations on the maternal behaviour of free-ranging domestic pigs. Applied Animal Behaviour Science, 16, 131–142. doi:10.1016/0168-1591(86)90105-X.
- Jensen, P. & Redbo, I. (1987). Behaviour during nest leaving in free-ranging domestic pigs. Applied Animal Behaviour Science, 18, 355–362. doi:10.1016/0168-1591(87)90229-2.
- Ko, H. L., Chongb, Q., Escribanoa, D., Camerlink, I., Mantecaa, X. & Lloncha, P. (2020). Pre-weaning socialization and environmental enrichment affect life-long response to regrouping in commercially-reared pigs. Applied Animal Behaviour Science, 229, 105044.
- Kobek-Kjeldager, C., Moustsen, V. A., Theil, P. K. & Pedersen, L. J. (2020). Effect of litter size, milk replacer and housing on behaviour and welfare related to sibling competition in litters from hyper-prolific sows. Applied Animal Behaviour Science, 230, 105032. doi:10.1016/j.applanim.2020.105032.
- Kutzer, T., Bünger, B., Kjaer, J. B. & Schrader, L. (2009). Effects of early contact between non-littermate piglets and of the complexity of farrowing conditions on social behaviour and weight gain. Applied Animal Behaviour Science, 121, 16–24. doi:10.1016/j.applanim.2009.08.004.
- Løvendahl, P., Damgaard, L. H., Nielsen, B. L., Thodberg, K., Su, G. & Rydhmer, L. (2005). Aggressive behaviour of sows at mixing and maternal behaviour are heritable and genetically correlated traits. Livestock Production Science, (93), 73–85. doi:10.1016/j.livprodsci.2004.11.008.
- Lundahl, I. (2019). Differences in nursing behavior comparing Swedish and Dutch Yorkshire breeds – in nursing pens where the piglets have access to the sow and piglets in the neighbor nursing pen. Bachelor thesis, Department of Animal Environment and Health, Swedish University of agricultural sciences. Available at: http://urn.kb.se/resolve?urn = urn:nbn:se:slu:epsilon-s-11024.
- Lundeheim, N. (2017). The rise and fall of Swedish pig breeding - pros and cons with genes from abroad. XVIII Baltic Animal Breeding Conferene, May 30–31, Lithuania.
- Matthews, S. G., Miller, A. L., Clapp, J., Plötz, T. & Kyriazakis, I. (2016). Early detection of health and welfare compromises through automated detection of behavioural changes in pigs. The Veterinary Journal, 217, 43–51. doi:10.1016/j.tvjl.2016.09.005.
- McGlone, J. J. (1985). A quantitative ethogram of aggressive and submissive behaviors in recently regrouped pigs. Journal of Animal Science, 61, 559–565. doi:10.2527/jas1985.613556x.
- Newberry, R. C., Wood-Gush, D. G. M. & Hall, J. W. (1988). Playful behaviour of piglets. Behavioural Processes, 17, 205–216. doi:10.1016/0376-6357(88)90004-6.
- Oostindjer, M., van den Brand, H., Kemp, B. & Bolhuis, J. E. (2011). Effects of environmental enrichment and loose housing of lactating sows on piglet behaviour before and after weaning. Applied Animal Behaviour Science, 134, 31–41. doi:10.1016/j.applanim.2011.06.011.
- Peden, R. S. E., Turner, S. P., Boyle, L. A. & Camerlink, I. (2018). The translation of animal welfare research into practice: the case of mixing aggression between pigs. Applied Animal Behaviour Science, 204, 1–9. doi:10.1016/j.applanim.2018.03.003.
- Petersen, H. V., Vestergaard, K. & Jensen, P. (1989). Integration of piglets into social groups of free-ranging domestic pigs. Applied Animal Behaviour Science, 23, 223–236. doi:10.1016/0168-1591(89)90113-5.
- Rydhmer, L. (2020). Advances in understanding the genetics of pig behaviour. In S. Edwards (Ed.), Understanding the Behaviour and Improving the Welfare of Pigs (1st ed.) (pp. 3–26). London: Burleigh Dodds Science Publishing). doi:10.1201/9781003048220.
- Salazar, L. C., Ko, H. L., Yang, C. H., Llonch, L., Manteca, X., Camerlink, I. & Llonch, P. (2018). Early socialisation as strategy to increase piglets’ social skills in intensive farming conditions. Applied Animal Behaviour Science, 206, 25–31. doi:10.1016/j.applanim.2018.05.033.
- Schrey, L., Kemper, N. & Fels, M. (2019). Behaviour and skin injuries of piglets originating from a novel group farrowing system before and after weaning. Agriculture, 9, 93. doi:10.3390/agriculture9050093.
- Singh, C., Verdon, M., Cronin, G. M. & Hemsworth, P. H. (2017). The behaviour and welfare of sows and piglets in farrowing crates or lactation pens. Animal, 11, 1210–1221. doi:10.1017/S1751731116002573.
- Turner, S., D’Eath, R., Roehe, R. & Lawrence, A. (2010). Selection against aggressiveness in pigs at re-grouping: practical application and implications for long-term behavioural patterns. Animal Welfare, 19(S1), 123–132. doi:10.1017/S0962728600002323.
- Vahlberg, J. (2019). Difference in health and behaviour between two different pig line crosses. Master of Science thesis, Department of Animal Breeding and Genetics, Swedish University of Agricultural Sciences. Available at: http://urn.kb.se/resolve?urn = urn:nbn:se:slu:epsilon-s-10300.
- Van Kerschaver, C., Turpin, D., Michiels, J. & Pluske, J. (2023). Reducing weaning stress in piglets by pre-weaning socialization and gradual separation from the sow: a review. Animals, 13, 1644. doi:10.3390/ani13101644.
- Van Putten, G. & Bure, R. G. (1997). Preparing gilts for group housing by increasing their social skills. Applied Animal Behaviour Science, 54, 173–183.
- Wattanakul, W., Stewart, A. H., Edwards, S. A. & English, P. R. (1997). Effects of grouping piglets and changing sow location on suckling behaviour and performance. Applied Animal Behaviour Science, 55, 21–35. doi:10.1016/S0168-1591(97)00020-8.
- Wechsler, B. (1996). Rearing pigs in species-specific family groups. Animal Welfare, 5, 25–35.
- Wilson, R., Holyoake, P., Cronin, G. & Doyle, R. (2014). Managing animal wellbeing: a preliminary survey of pig farmers. Australian Veterinary Journal, 92, 206–212. doi:10.1111/avj.12169.