ABSTRACT
This study describes the isolation of various marine bacteriafrom sponges collected from the Red Sea (Saudi Arabia) andL-asparaginase (anti-cancer enzyme) production from bacterialisolates. The 16S rDNA based phylogenetic analysis revealed thatthe isolate WSA3 was a Bacillus subtilis. Its partial-length genesequence was submitted to GenBank under the accession numberMK072695. The new B. subtilis strain harbored the exact size(1128 bp) of the new L-asparaginase (ansZ) gene as confirmedby PCR and in gel visualization, which was submitted to the NCBIdatabase (accession number MN566442). The molecular weightof partially purified L-asparaginase was determined as 45 kDa bySDS-PAGE. In addition, the enzyme L-asparaginase did not showglutaminase activity which is very important from a medical pointof view. Moreover, 100 μg/mL of the partially purified B. subtilis Lasparaginaseshowed promising anti-cancer activities when testedagainst three cancer cell lines (HCT-116, MCF-7, and HepG2).
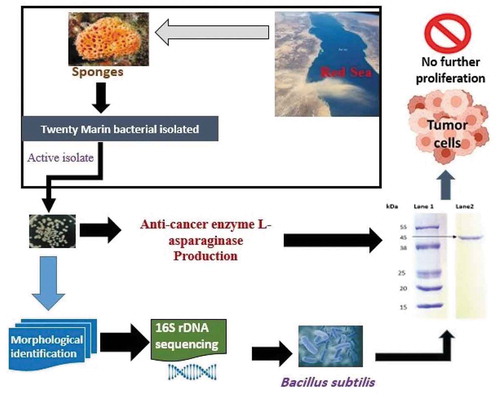
Graphical abstract
L-asparaginase (EC.3.5.1.1; L-asparagine amidohydrolase) is an anti-cancer enzyme which catalyzes the conversion of L-asparagine to produce aspartate and ammonia [Citation1]. This enzyme is medically used for treating leukemia and non-Hodgkin lymphomas [Citation2]. The anti-cancerous activity of L-asparaginase is due to its ability to remove L-asparagine from blood serum which is required by malignant cells to maintain their growth. L-asparaginase is also used to reduce the formation of the suspected human carcinogen acrylamide in heat-treated foods [Citation3].
L-asparaginase has been found to produce by various microorganisms including bacteria, actinomycetes, fungi, and plants, algae and animals [Citation4,Citation5]. However, microorganisms, such as fungi, bacteria, yeast, actinomycetes and algae, represent a preferred source of asparaginase enzyme because they can be easily cultivated. Among the microbes, the L-asparaginase produced by Erwinia carotovora and Escherichia coli has been studied the most due to its antineoplastic action [Citation6,Citation7]. Despite the potential benefits of bacterial L-asparaginase, some evidences suggested toxicity of microbially produced L-asparaginase in some patients [Citation8]. In addition, the current treatments with L-asparaginase lead to potential side effects and allergic responses [Citation9]. Such undesired effects may be avoided by using L-asparaginase isolated from new microorganisms [Citation10,Citation11]. Therefore, novel sources of L-asparaginase may have the advantage of being alternative to available anti-tumor agents with lower or nil side effects. Different types of cancers have been the leading causes of human death globally [Citation12]. To control or diminish the mortality due to cancer, research is being done to identify novel, affordable, reliable and less toxic chemotherapeutic alternatives against a broad spectrum of cancer. However, increasing incidences of drug resistance in tumors, inadequate drug internalization, and complications associated with chemotherapy are some of the reasons that further complicates the route to a successful and nontoxic treatment [Citation13,Citation14]. Cancerous cells may express a highly dynamic but complex cellular environment that is sometimes caused by the subtle alterations in the matrix composition modulating its stiffness [Citation15,Citation16]. Due to the complexity and high mortality rate, there is an urgent need to opt new strategies for cancer treatment, for which the researchers from around the world are making their efforts. To fulfill this demand, discoveries of various enzymes augmenting the reduction of cancer progression either directly or indirectly by efficiently having antagonistic interactions with abroad range of cancer types has opened up new vistas [Citation17]. Among those enzymes, L-asparaginase has emerged as a promising cure to combat the obstacles in cancer management by massively reducing the amount of asparagine. As an example, leukemic cells absorbs the amino acid asparagine from blood serum or may synthesize it by themselves but in a limited amount [Citation11]. The higher demand of asparagine by cancerous cells becomes the target of L-asparaginase and it depletes the pool of L-asparagine and succumb the cancerous cells to death. Other important factors for the enzyme L-asparaginase to be used as key anticancer enzymes could be no immunogenic complication and side effects, high affinity toward L-asparagine, and longer persistence in plasma [Citation18].
In this regard, marine microorganisms could be worth exploring since they produce compounds with unique biological characteristics as a result of their adaptation to the harsh conditions of marine ecosystems [Citation3,Citation19].Therefore, the present study was systematically designed to accomplish the following objectives- (i) isolation and identification of new L-asparaginase-producing marine bacteria from marine sponges, (ii) screening of L-asparaginase activity, (iii) optimization of L-asparaginase production, (iv) partial purification of L-asparaginase and identification of L-asparaginase encoding gene (ansZ), and (v) anticancer activity of L-asparaginase to three different cancer cell lines
Materials and methods
Collection and isolation of bacteria from marine sponges
The sponges belonging to Hyrtios erectus, Xestospongia testudinaria, Callyspongia siphonella and Callyspongia sp. were collected from the West Coast of the Red Sea, Saudi Arabia, by scuba diving to the depth of approximately three meters. The collected sponge samples were kept in sterile screw-capped bottles and taken immediately to the laboratory for the selective isolation of L-asparaginase-producing bacteria. The samples were further processed following the of Pradhan et al. [Citation20] with some modifications. Briefly, the sponges were rinsed with sterile seawater and the middle portion of the sponge tissue (1 cm3) was cut using a sterile knife. Afterward, the sponge tissues were left at room temperature (24 ± 2°C) for 5 min under aseptic conditions. The isolation of bacteria from the collected sponges was performed as described by Dharmaraj [Citation21] with minor modifications. In brief, the sponge extracts were prepared by squeezing the sponges gently with a glass stick. Then, 1 mL of each sponge extract was tem times diluted with sterilized sea water. Afterward, a total of 1 mL diluted extract was taken out and spread over marine agar medium containing the following ingredients: peptone (5 g/L), yeast extract (1 g/L), ferric citrate (0.1 g/L), sodium chloride (19.45 g/L), magnesium chloride (8.8 g/L), sodium sulfate (3.24 g/L), calcium chloride (1.8 g/L), potassium chloride (0.55 g/L), sodium bicarbonate (0.16 g/L), potassium bromide (0.08 g/L), strontium chloride (0.034 g/L), boric acid (0.022 g/L), sodium silicate (0.004 g/L), sodium fluoride (0.0024 g/L), ammonium nitrate (0.0016 g/L), disodium phosphate (0.008 g/L), agar (15 g/L), final pH (7.6 ± 0.2).. Following the incubation at room temperature (24 ± 2°C) for 48 h, the morphologically distinct colonies were picked out, purified by the streak plate technique, and maintained at 4°C until further use.
Screening for L-asparaginase producing marine bacteria
The morphologically distinct colonies were streaked on sterile plates containing modified M9 medium (Na2HPO4 · 2H2O 6.0 g/L; KH2PO4 3.0 g/L; MgSO4.7H2O 0.5 g/L; NaCl- 0.5 g/L; CaCl2.2H2O 0.014 g/L; glucose 2% w/v; L-asparagine 5.0 g/L; agar 20 g/L) supplemented with Phenol Red (2.5%) as a pH indicator. Control plates consisted of uninoculated medium. The plates were incubated at 37 ± 2°C for 24–72 h [Citation22]. L-asparaginase activity was identified by the change in the color of the medium from yellow to pink due to the change in pH. The L-asparaginase positive colonies were further purified by streaking techniques on nutrient agar plates. The purified cultures were maintained at 4°C for further studies.
Molecular identification of the selected isolate
The genomic DNA of the L-asparaginase producing isolate was extracted by using QIAamp DNA Mini kit (Qiagen Inc., Valencia, CA) following manufacturer’s instructions. For this, overnight grown bacterial cultures were used as templates for the amplification of 16S rRNA gene sequence using the universal primers −5′ CCA GCA GCC GCG GTA ATA CG 3′/5′ ATC GG(C/T) TAC CTT GTT ACG ACT TC 3′ designed originally by Lu et al [Citation23]. The polymerase chain reaction (PCR) was performed based on the following program: 10 min of denaturation (94°C), followed by 35 cycles of 1 min denaturation (94°C), 1 min annealing (55°C), 2 min extension (72°C) and a final extension for 10 min (72°C). Subsequently, the amplified PCR product was examined by running 1.2% (w/v) agarose gel electrophoresis, purified and further used for DNA sequencing. Thereafter, the DNA sequence was compared for similarity check among the sequences of GenBank (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Phylogenetic trees were constructed by neighbor joining method of MEGA (version 5.0) with bootstrap analyses for 1000 replicates.
Optimization of asparaginase activity
Production of L-asparaginase was allowed in in 250 mL sterile glass flasks containing 100 mL of M9 broth basal medium (pH 7.0 ± 0.2) consisting of (g/L): Na2HPO4.2H2O (6.0), KH2PO4 (3.0), NaCl (0.5), L-methionine (1.0), MgSO4.7H2O (0.25), CaCl2 (0.014) and glucose (2.0). The medium was seeded with 10 mL inoculum of the bacterial strain. The effect of different incubation and nutrition parameters on asparaginase activity such as static/shaken incubation conditions, carbon source, nitrogen source and amino acid source was studied. Firstly, the selected isolate was incubated under static and shaken (150 rpm) conditions. Then, the best incubation condition was chosen and different carbon sources (e.g. lactose, glucose, fructose, glycerol, sucrose and galactose) at a concentration of 1% (w/v) were tested. Afterward, the carbon source enhancing L-asparaginase activity maximally was chosen and then, effect of different nitrogen sources (e.g. asparagine, yeast extract, peptone and tryptone) at a fixed concentration of 0.5% (w/v) on L-asparaginase activity were investigated. Subsequently, the best one among the nitrogen sources was selected and different amino acid sources (e.g. arginine, glutamine, glycine and methionine) at a concentration of 0.5% (w/v) were examined. The best combination of all these nutrient sources and incubation giving optimum production of L-asparaginase was selected. All experiments were performed in duplicate at 35 ± 2°C for 48 h.
Analytical determinations
L-asparaginase activity was determined according to the method described by Ramaiah and Chandramohan [Citation24]. Briefly, the reaction mixture consisted of 100 μL of enzyme extract, 100 μL of 0.05 M Tris-HCl buffer (pH 8.6) and 1.7 mL of 0.01 M L-asparagine was incubated at 37°C for 10 min. The reaction was stopped by the addition of 50 µL of 1.5 M tri-chloroacetic acid (TCA) and the precipitated protein was removed by centrifugation (at 6000 × g for 5 min at 4°C) and release of ammonia was determined spectrophotometrically at 450 nm by nesslerization. The reaction mixture without the enzyme extract was run in parallel as a control. One unit (U) of L-asparaginase activity was defined as the amount of enzyme that catalyzes the formation of 1 µmol of ammonia per minute under the assay conditions and expressed in U/mL.
Glutaminase activity was measured according to the method of Imada et al. [Citation25]. The reaction mixture consisted of 0.5 mL of enzyme extract, 0.5 mL of 0.04 M L-glutamine, 0.5 mL of 0.05 M Tris-HCl buffer (pH 7.4) and 0.5 mL of distilled water. Following the incubation for 30 min at 37° C, a 0.5 mL of 1.5 M TCA was added to stop the reaction. The samples were centrifuged at 5000 × g for 10 min and the absorbance of the supernatant was determined spectrophotometrically at 450 nm by nesslerization. The reaction mixture without the enzyme extract was used as a control. One unit (U) of glutaminase activity was defined as the amount of enzyme that catalyzes the formation of 1 µmol of ammonia per minute under the assay conditions and expressed in U/mL. The Lowry’s method [Citation26] was followed to determine the total protein concentration in samples using bovine serum albumin (BSA) as a standard.
Partial purification of L-asparaginase
L-asparaginase was partially purified from the crude enzyme extract by ammonium sulfate (85%) precipitation. The precipitate enzyme was resuspended in a minimal volume of 1 M Tris HCl (pH 7.5) and dialyzed overnight through a semi-permeable membrane against the same buffer at 4°C.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE)
The purity of asparaginase enzyme was determined by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS PAGE) according to the method of [Citation27]. For this, the partially purified enzyme fractions were resuspended in 200 µL of lysis buffer (62.5 mM Tris -HCl, pH 6.8), SDS (2%), glycerol (15%), 2-mercaptoethanol (5%) and Bromophenol Blue 0.001% (tracking dye). The samples were boiled for 5 min at 100°C and loaded onto a 15% SDS polyacrylamide gel and run at 100 V for 7–8 hours. After electrophoresis, the gel was stained with Coomassie Brilliant Blue R-250 and distained using a mixture of 500 mL methanol, 100 mL acetic acid and distilled water in a final volume of 1 L. The gel was visualized using a Gel Documentation System. The molecular weight of the partially purified L-asparaginase was determined in comparison with standard molecular weight markers (Sigma- Aldrich, US).
Asparaginase gene identification
Genomic DNA extraction
The selected bacterial isolate was grown overnight in Luria-Bertani medium. Then, aliquots of 10 mL of bacterial culture were centrifuged at 12,000 × g for 15 min and washed once in sterile distilled water. Genomic DNA isolation was done using a Genomic DNA Purification kit (Thermo Fisher Scientific, Massachusetts, USA).
Primers used for identification of asparaginase gene
The ansZ gene primers were prepared to identify the presence of L-asparaginase gene (ansZ). The primer was designed according to the sequence of ansZ gene of the closest species, e.g., Bacillus subtilis (NCBI accession number KF444946.1). The PCR was done in a thermal cycler, (Bibby Scientific, UK) for a total volume of 25 μL containing 2 μL of DNA, 1 μL of each primer, 12.5 μL master mix and 9.5 μL of sterile distilled water. The PCR conditions for gene amplification involved an initial denaturation for 5 min at 94°C, followed by a number of 35 cycles of denaturation for 1 min at 94°C, annealing for 1 min at 55°C and extension for 2 min at 72°C, then, a final extension cycle for 10 min at 72°C. Sanger sequencing was carried out at Beijing Genomic Institute (BGI), Hong Kong, China and the homology of the gene sequence carried out with ansZ gene sequence from NCBI BLASTx database.
Anticancer assay
The cytotoxicity assay was performed using colon cancer cell lines (HCT-116), breast cancer cell lines (MCF-7) and liver cancer cell lines (HepG2). The cell lines were maintained in Roswell Park Memorial Institute (RPMI) media containing 100 µg mL−1 streptomycin, 100 µg mL−1 penicillin and heat-inactivated fetal bovine serum (10%) in an incubator supplying 5% CO2 (v/v) at 37°C. The cytotoxicity of the partially purified asparaginase produced by the selected bacterial isolate was tested against exponentially growing cancer cells. Cancer cells were taken from RPMI media and cultured in 96-well plates containing 100 μL of trypsin-EDTA (0.25%). Afterward, the cells were treated with 0.0–100 μg/mL of the partially purified enzyme (negative controls no treatment of L-asparaginase and doxorubicin while only doxorubicin (20 µg mL−1) was used as a positive control and incubated for 48 h at 37°C. A 5 mg/mL of MTT (−3- (4, 5-Dimethylthiazol-2-yl)-2, 5- diphenyltetrazolium bromide) dye (10 μL/well) was added to the wells and incubated at 37°C in a CO2 incubator for 4 h. After incubation, liquid fraction was aspirated from the wells and the remaining crystals were dissolved in 150 μL DMSO which was measured spectrophotometrically at 570 nm using an ELISA reader (Bio Tek Instruments, Inc.).
Statistical analysis
Statistical analyses of data were performed as previously outlined by Freeman et al. [Citation28] and multiple comparisons were performed following the standard procedure of Duncan’s New Multiple Range test [Citation29].
Results
Collection and isolation of bacteria from sponges
The sponges Hyrtios erectus, Xestospongia testudinaria, Callyspongia siphonella and Callyspongia sp. (uncharacterized), were collected from the West Coast of the Red Sea, Saudi Arabia. Sponge extracts were prepared and serially diluted as indicated in section 2.1. The diluted samples were incubated on marine agar medium at room temperature (24 ± 2°C) for 48 h. After incubation, 20 morphologically distinct colonies were observed and used for further studies.
Screening of L- asparaginase producing microorganisms
Twenty bacterial isolates were streaked onto sterile Petri plates containing modified M9 medium supplemented with 2% glucose, 5 g/L asparagine and Phenol Red (2.5%). Among the twenty bacterial isolates, only one isolate designed as WSA 3 showed a pink zone around it ().
Table 1. Screening of L-asparaginase activity of isolated bacteria from marine sponges
Molecular identification
16S rRNA gene sequence analysis of the L-asparaginase producing isolate was performed by genomic DNA extraction, PCR amplification and sequencing. Then, the sequences were submitted to GenBank (Accession No. MK072695). Based on molecular identification, it was found that the selected isolate WSA3 belonged to Bacillus subtilis with 100% identity and 100% coverage as revealed by BLAST (). The closest two clusters to the new strain’s cluster also included bacterial strains of Bacillus subtilis followed by other clusters including strains of both B. subtilis and B. tequilensis ().
Table 2. BLAST analysis of partial 16S rDNA of the new Bacillus subtilis isolate (acc. no. MK072695) versus the most related taxa in the gene bank
Optimization of L-asparaginase production
Different parameters affecting L-asparaginase production such as static and shaken conditions, various sources of carbon (C), nitrogen (N) and amino acids were studied in order to maximize L-asparaginase production by the new B. subtilis isolate. It was found that shaken cultures (150 rpm) led to a 24.6% higher L-asparaginase production than static ones (). Among the different carbon sources tested, glucose and lactose were the most favorable ones for asparaginase production (5.3 and 3.8% higher than the control, respectively) (). Among the various nitrogen sources assayed tryptone and L-asparagine produced the highest L-asparaginase levels (5.6 and 33.5% increased, respectively, compared to the control) (). As for the amino acids, glutamine led to the highest L-asparaginase level (21.6% higher over control) ().
Asparaginase enzyme partial purification
Glutaminase-free L-asparaginase enzyme from the new B. subtilis isolate was partially purified by ammonium sulfate precipitation. The enzyme was purified by 3-fold with a yield of 49.7% (). The SDS-PAGE of the partially purified enzyme showed a molecular weight of 45 kDa ().
Table 3. Purification of L-asparaginase from the new Bacillus subtilis isolate
Asparaginase gene identification
The partially purified L-asparaginase enzyme produced by the B. subtilis isolate contained the specific asparaginase enzyme producing gene ansZ. PCR analysis confirmed that the new B. subtilis strain harbored the correct size (1128 bp) of the L-asparaginase (ansZ) gene yielding a number of 376 deduced amino acids (). The length of its polypeptide chain was identical to that of the B. subtilis strain B11-06 with new ansZ gene was submitted to the NCBI and given the accession no. MN566442.
Anti-cancer activity
For the anticancer assay, the partially purified L-asparaginase enzyme of the new B. subtilis isolate was treated against MCF-7, HCT-116 and HepG-2 human cancer cell lines (). The cytotoxicity studies shown that the partially purified enzyme (100 μg/mL) presented significant anti-cancer activity (percent reduction over negative control) against MCF-7 (65.00 ± 0.40), HCT-116 (67.00 ± 0.42) and HepG-2 (68.00 ± 0.39) cancer cells than the doxorubicin positive controls (46.07 ± 0.40, 50.40 ± 0.22 and 43.00 ± 0.12, respectively) ().
Table 4. Anticancer activity assay (MTT assay) of purified asparaginase enzyme (absorbance 570 nm) against various human cell lines
Discussion
Commercially available asparaginase of microbial origin can produce side effects such as allergies and toxicity during tumor therapy. Consequently, new microbial asparaginase sources are required. In the present paper, 20 sponge-associate bacteria were isolated from the Red Sea, Saudi Arabia, which is regarded as a bountiful source of bacterial species [Citation30]. From the 20 isolates only one was able to produce L-asparaginase which was further identified as Bacillus subtilis. Asparaginase enzymes have been already found in several marine bacteria, Bacillus species being one of the most abundant ones [Citation31] due to its resistance to extreme environments [Citation32]. Similar to our findings, Mostafa et al. have also reported the isolation of L-asparaginase producing B. velezensis from marine sediments which exhibited anticancer activity against MCF-7 cell line [Citation33]. In another study, Alrumman et al. [Citation34] also isolated B. licheniformis from red sea, Saudi Arabia and extracted L-asparaginase enzyme which showed promising anticancer activity [Citation34].
Bacterial culture conditions significantly affect the L-asparaginase production [Citation35]. Due to this fact, variable growth conditions were optimized to maximally increase the yield of L-asparaginase production. According to the results obtained, shaking conditions, nitrogen sources and amino acid sources were the parameters that affected the L-asparaginase production most significantly. Shaken cultures led to a higher L-asparaginase production than static ones due to agitation improves the rate of oxygen transfer [Citation36]. However, if agitation is too high asparaginase production diminishes due to shear forces [Citation36]. Also, it is known that L-asparaginase production is nitrogen-regulated [Citation37]. No glutaminase activity was found in the L-asparaginase obtained from the new B. subtilis isolate, which makes it superior than the commercially available asparaginases which shows glutaminase activity causing diverse severe health problems to patients during anti-cancer therapy [Citation38]. This activity of L-asparaginase limits its use in chemotherapy because it is not able to distinguish between the chemical structures of glutamine and asparagine. Both the amino acids carry an amide group, however, glutamine has an additional methyl (-CH3) group [Citation39]. Glutaminase-free L-asparaginases have also been reported from other Bacillus species [Citation40–42]. In addition, the partially purified glutaminase-free L-asparaginase obtained from cultures of the new B. subtilis isolate showed significant anti-cancer activity against colon, breast and liver cancer cell lines. Asparagine can be supplied to cancer cells by two sources: (i) synthesis of asparagine by cancer cells or (ii) uptake of asparagine from blood stream. However, the presence of L-asparaginase, L-asparagine is hydrolyzed into ammonia (NH3) and aspartic acid (). Thus, cancer cells are forced to grow without L-asparagine which leads to their death. Interestingly, the activity does not affect the growth of normal cells of the body, normal cells managed to survive due to very low demand of L-asparagine. The split of L-asparagine into NH3 and L-aspartate by L-asparaginase is achieved by a “Ping Pong” type or double displacement mechanism. Here, the nucleophilic group (Nuc) of L-asparaginase interacts with the Cγ of L-asparagine and two intermediates are formed (i) a tetrahedral intermediate, which is then converted to an (ii) acyl-enzyme intermediate. During this process NH3 is released. Subsequently, in the presence of water molecules, aspartic acid or aspartate is formed releasing the enzyme L-asparaginase with Nuc group [Citation43]. Overall, our results are highly interesting and paves the ways for development of drug based on L-asparaginase extracted from microbes isolated from new locations. This points out bacteria from The Red Sea as promising sources of L-asparaginase enzymes to be applied as anti-cancer drugs.
Conclusion
In conclusion, this study demonstrated that the new B. subtilis bacterium isolated from marine sponges can be a good source for glutaminase-free L-asparaginase production for the development of anti-cancer drugs with low or no side-effects. However, much research is still required to assess this possibility.
Authorship contribution statement
All authors contributed to data analysis, drafting, or revising the manuscript. All authors approved the final manuscript and are accountable for all aspects of the work.
Acknowledgments
The authors extend their appreciation to the Deputyship for Research & Innovation, “Ministry of Education” in Saudi Arabia for funding this research work through the project number IFKSURG-1438-029.
Disclosure statement
The authors declare that they have no conflict of interest.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Bhargavi M, Jayamadhuri R. Isolation and screening of marine bacteria producing anti-cancer enzyme L-asparaginase. Am J Mar Sci. 2016;4:1–3.
- Müller HJ, Boos J. Use of L-asparaginase in childhood ALL. Crit Rev Oncol Hematol. 1998;28:97–113.
- Qeshmi FI, Homaei A, Fernandes P, et al. Marine microbial L-asparaginase: biochemistry, molecular approaches and applications in tumor therapy and in food industry. Microbiol Res. 2018;208:99–112.
- Ali U, Naveed M, Ullah A, et al. L-asparaginase as a critical component to combat Acute Lymphoblastic Leukaemia (ALL): A novel approach to target ALL. Eur J Pharmacol. 2016. Epub ahead of print. DOI:10.1016/j.ejphar.2015.12.023.
- El-Naggar NEA, Deraz SF, Soliman HM, et al. Purification, characterization, cytotoxicity and anticancer activities of L-asparaginase, anti-colon cancer protein, from the newly isolated alkaliphilic Streptomyces fradiae NEAE-82. Sci Rep. 2016. Epub ahead of print. DOI:10.1038/srep32926.
- Belén LH, Lissabet JB, de Oliveira Rangel-yagui C, et al. A structural in silico analysis of the immunogenicity of L-asparaginase from Escherichia coli and Erwinia carotovora. Biologicals. 2019;59:47–55.
- Pourhossein M, Korbekandi H. Cloning, expression, purification and characterisation of Erwinia carotovora L-asparaginase in Escherichia coli. Adv Biomed Res. 2014. Epub ahead of print. DOI:10.4103/2277-9175.127995
- Sharma D, Singh K, Singh K, et al. Insights into the microbial L-Asparaginases: from production to practical applications. Current Protein Pept Sci. 2019;20:452–464.
- Hijiya N, Van Der Sluis IM. Asparaginase-Associated toxicity in children with acute lymphoblastic leukemia. Leukemia Lymphoma. 2016;57(4):748–757.
- Muneer F, Siddique MH, Azeem F, et al. Microbial l-asparaginase: purification, characterization and applications. Arch Microbiol. 2020. Epub ahead of print. DOI:10.1007/s00203-020-01814-1.
- Narta UK, Kanwar SS, Azmi W. Pharmacological and clinical evaluation of L-asparaginase in the treatment of leukemia. Crit Rev Oncol Hematol. 2007;61:208–221.
- Carioli G, Malvezzi M, Bertuccio P, et al. Cancer mortality in the elderly in 11 countries worldwide, 1970–2015. Ann Oncol. 2019. Epub ahead of print. DOI:10.1093/annonc/mdz178.
- Leary M, Heerboth S, Lapinska K, et al. Sensitization of drug resistant cancer cells: A matter of combination therapy. Cancers (Basel). 2018. Epub ahead of print. DOI:10.3390/cancers10120483.
- Petrillo A, Pompella L, Tirino G, et al. Perioperative treatment in resectable gastric cancer: current perspectives and future directions. Cancers (Basel). 2019. Epub ahead of print. DOI:10.3390/cancers11030399.
- Wang M, Zhao J, Zhang L, et al. Role of tumor microenvironment in tumorigenesis. J Cancer. 2017. Epub ahead of print. DOI:10.7150/jca.17648.
- Baghban R, Roshangar L, Jahanban-Esfahlan R, et al. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun Signaling. 2020. Epub ahead of print. DOI:10.1186/s12964-020-0530-4.
- Baig MH, Adil M, Khan R, et al. Enzyme targeting strategies for prevention and treatment of cancer: implications for cancer therapy. Semin Cancer Biol. 2019. Epub ahead of print. DOI:10.1016/j.semcancer.2017.12.003.
- Parmentier JH, Maggi M, Tarasco E, et al. Glutaminase activity determines cytotoxicity of l-asparaginases on most leukemia cell lines. Leuk Res. 2015. Epub ahead of print. DOI:10.1016/j.leukres.2015.04.008.
- Oza VP, Parmar PP, Patel DH, et al. Cloning, expression and characterization of l-asparaginase from Withania somnifera L. for large scale production. 3 Biotech. 2011;1:21–26.
- Pradhan B, Dash SK, Sahoo S. Screening and characterization of extracelluar L-asparaginase producing Bacillus subtilis strain hswx88, isolated from Taptapani hotspring of Odisha, India. Asian Pac J Tropical Biomedicine. 2013;3:936–941.
- Dharmaraj S. Study of L-asparaginase production by Streptomyces noursei MTCC 10469, isolated from marine sponge Callyspongia diffusa. Iran J Biotechnol. 2011;9:102–108.
- Gulati R, Saxena RK, Gupta R. A rapid plate assay for screening l-asparaginase producing micro-organisms. Lett Appl Microbiol. 1997;24:23–26.
- Lu JJ, Perng CL, Lee SY, et al. Use of PCR with universal primers and restriction endonuclease digestions for detection and identification of common bacterial pathogens in cerebrospinal fluid. J Clin Microbiol. 2000;38:2076–2080.
- Ramaiah N, Chandramohan D. Production of L-asparaginase by the marine luminous bacteria. Indian J Mar Sci. 1992;21:212.
- Imada A, Igarasi S, Nakahama K, et al. Asparaginase and glutaminase activities of micro-organisms. Microbiology. 1973;76:85–99.
- Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275.
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. nature. 1970;227:680–685.
- Freeman GH, Gomez KA, Gomez AA. Statistical procedures for agricultural research. New York, NY: John Wiley & Sons; 1985. Epub ahead of print. DOI:10.2307/2530673.
- Duncan DB. Multiple range and multiple F tests. Biometrics. 1955;11:1–42.
- Abdelfattah MS, Elmallah MIY, Hawas UW, et al. Isolation and characterization of marine-derived actinomycetes with cytotoxic activity from the Red Sea coast. Asian Pac J Tropical Biomedicine. 2016;6:651–657.
- Izadpanah Qeshmi F, Javadpour S, Malekzadeh K, et al. Persian gulf is a bioresource of potent L-asparaginase producing bacteria: isolation & molecular differentiating. Int J Environ Res. 2014;8(3):813-818.
- Nicholson WL, Munakata N, Horneck G, et al. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol Mol Biol Rev. 2000;64:548–572.
- Mostafa Y, Alrumman S, Alamri S, et al. Enhanced production of glutaminase-free L-asparaginase by marine Bacillus velezensis and cytotoxic activity against breast cancer cell lines. Electron J Biotechnol. 2019;42:6–15.
- Alrumman SA, Mostafa YS, Al-izran KA, et al. Production and anticancer activity of an L-Asparaginase from Bacillus licheniformis isolated from the Red Sea, Saudi Arabia. Sci Rep. 2019. Epub ahead of print. DOI:10.1038/s41598-019-40512-x.
- Hymavathi M, Sathish T, Rao CS, et al. Enhancement of L-asparaginase production by isolated Bacillus circulans (MTCC 8574) using response surface methodology. Appl Biochem Biotechnol. 2009;159:191–198.
- Devi S, KULSHRESHTHA A, AK RAI, et al. Bench-scale production of L-asparaginase from Erwinia carotovora in a laboratory fermenter. Int J Life Sci Pharm Res. 2012;3:25–35.
- Wakil SSM, Adelegan AA. Screening, production and optimization of L-asparaginase from soil bacteria isolated in Ibadan, South-western Nigeria. J Basic Appl Sci. 2015;11:39–51.
- Koprivnikar J, McCloskey J, Faderl S. Safety, efficacy, and clinical utility of asparaginase in the treatment of adult patients with acute lymphoblastic leukemia. Onco Targets Ther. 2017;10:1413.
- Ramya LN, Doble M, Rekha VPB, et al. L-asparaginase as potent anti-leukemic agent and its significance of having reduced glutaminase side activity for better treatment of acute lymphoblastic leukaemia. Appl Biochem Biotechnol. 2012. Epub ahead of print. DOI:10.1007/s12010-012-9755-z.
- Mahajan RV, Saran S, Kameswaran K, et al. Efficient production of L-asparaginase from Bacillus licheniformis with low-glutaminase activity: optimization, scale up and acrylamide degradation studies. Bioresour Technol. 2012;125:11–16.
- Mahajan RV, Kumar V, Rajendran V, et al. Purification and characterization of a novel and robust L-asparaginase having low-glutaminase activity from Bacillus licheniformis: in vitro evaluation of anti-cancerous properties. PLoS One. 2014;9:e99037.
- Sudhir AP, Agarwaal VV, Dave BR, et al. Enhanced catalysis of L-asparaginase from Bacillus licheniformis by a rational redesign. Enzyme Microb Technol. 2016;86:1–6.
- Shrivastava A, Khan AA, Khurshid M, et al. Recent developments in l-asparaginase discovery and its potential as anticancer agent. Crit Rev Oncol Hematol. 2016. Epub ahead of print. DOI:10.1016/j.critrevonc.2015.01.002.





