ABSTRACT
Resveratrol is a natural polyphenol with beneficial antioxidant properties. It suppresses the migration of osteoblast-like MC3T3-E1 cells induced by epidermal growth factor, via SIRT1-mediated inhibition of SAPK/JNK and Akt. Moreover, insulin-like growth factor-I (IGF-I) stimulates the migration involving the pathways of p44/p42 mitogen-activated protein (MAP) kinase and Akt. Therefore, we investigated the effects of resveratrol on IGF-I-induced cell migration. Resveratrol and SRT1720, an activator of SIRT1, suppressed IGF-I-induced migration. Inauhzin, a SIRT1 inhibitor, significantly rescued the inhibition of IGF-I-induced cell migration by resveratrol. Resveratrol inhibited IGF-I-induced phosphorylation of p44/p42 MAP kinase but not Akt. SRT1720 inhibited IGF-I-induced phosphorylation of p44/p42 MAP kinase. Furthermore, PD98059, p44/p42 MAP kinase inhibitor, alone suppressed IGF-I-induced osteoblast migration, but did not affect the suppressive effect of resveratrol when administered concomitantly. These findings strongly suggest that resveratrol suppresses IGF-I-induced osteoblast migration via SIRT1 activation at least partially by attenuating the p44/p42 MAP kinase pathway.
Graphical abstract
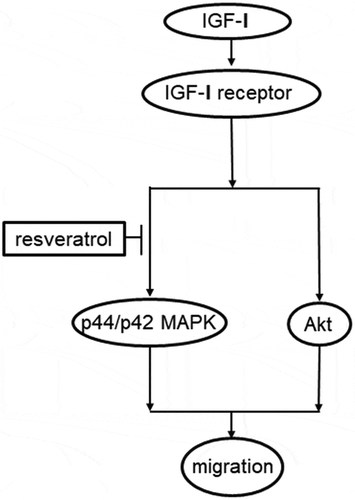
Schematic illustration of the mechanism underlying the effects of resveratrol on the IGF-I-induced migration of osteoblast-like MC3T3-E1cells.
Resveratrol is a natural polyphenolic compound found in grapes, berries, and red wine [Citation1]. Recent studies have suggested that resveratrol possesses various pharmacological benefits in human health, including anti–inflammatory and antioxidant effects, against cancer, diabetes, and cardiovascular and neurodegenerative diseases [Citation1–3]. The French population tend to consume a lot of meals with saturated fatty acids; however, circulating levels of saturated fatty acids remain relatively low, and mortality rates associated with coronary heart disease are low in this population compared to those in other countries associated with a similar lifestyle [Citation4]. This remarkable phenomenon, called the French paradox, is associated with the moderate consumption of red wine, which contains high levels of resveratrol [Citation4,Citation5]. The favorable effect of red wine on bone health has also been evidenced by the risk of hip fracture being reportedly lower in women who prefer to consume wine than in nondrinkers [Citation6].
Bone metabolism is a regenerative process in which old bone tissue is removed from the skeleton and new bone tissue is formed [Citation7]. This process, also called as bone remodeling, is finely conducted by two types of bone cells, osteoclasts, and osteoblasts [Citation7]. As bone quality and quantity is adequately coordinated by this process, the dysfunction of bone metabolism leads to remarkable pathological conditions such as osteoporosis and fracture healing distress [Citation8]. Insulin-like growth factor-I (IGF-I), normally embedded in the bone matrix, plays a pivotal role in the regulation of bone remodeling and homeostasis [Citation9,Citation10]. Low serum concentrations of IGF-I have been associated with a higher incidence of osteoporosis and osteoporotic fractures in postmenopausal females [Citation11,Citation12]. Regarding the effects on osteoblasts, we have previously reported that IGF-I stimulates mature osteoblast phenotype alkaline phosphatase (ALP) activity via the activation of both p44/p42 mitogen-activated protein (MAP) kinase and phosphatidylinositol 3 (PI3)kinase/Akt in osteoblast-like MC3T3-E1 cells [Citation13,Citation14]. We also showed that IGF-I induces osteoblast migration, an important step for bone remodeling and fracture repair [Citation15], whereby the process is mediated through the activation of p44/p42 MAP kinase in addition to phosphatidylinositol 3-kinase/Akt [Citation16].
Resveratrol reportedly leads to osteoblast differentiation in vitro [Citation17]. Regarding the mechanism of resveratrol activity, the activation of sirtuin 1 (SIRT1), a nicotinamide adenine dinucleotide-dependent histone deacetylase, could be involved in several effects [Citation18,Citation19]. We recently reported that resveratrol remarkably suppresses the epidermal growth factor (EGF)-induced migration of osteoblast-like MC3T3-E1 cells through the inhibition of SAPK/JNK and Akt pathways in part via SIRT1 [Citation20]. However, the precise mechanisms underlying the effect of resveratrol on osteoblast migration have not yet been elucidated. In the present study, we investigated the effect of resveratrol on the IGF-I-induced migration of osteoblast-like MC3T3-E1 cells. We found that resveratrol suppresses IGF-I-induced osteoblast migration through the attenuation of the p44/p42 MAP kinase pathway, and that the effect is mediated via SIRT1 activation.
Materials and methods
Materials
IGF-I was obtained from R&D Systems, Inc. (Minneapolis, MN, USA). Resveratrol and SRT1720 were obtained from EMD Millipore (Billerica, MA, USA). PD98059 and inauhzin were obtained from Calbiochem-Novabiochem Corp. (San Diego, CA, USA). Phospho-specific p44/p42 MAP kinase antibodies, p44/p42 MAP kinase antibodies, phospho-specific Akt antibodies (Thr308), and Akt antibodies were purchased from Cell Signaling Technology, Inc. (Beverly, MA, USA) and used as primary antibodies. The ECL western blot detection system was obtained from GE Healthcare Life Sciences (Chalfont, UK). All other materials and chemicals were purchased from commercial sources. Resveratrol and SRT1720 were dissolved in dimethyl sulfoxide [Citation20]. The maximum concentration of dimethyl sulfoxide was 0.1%, which did not affect the results of the cell migration assay or Western blotting [Citation20].
Cell culture
Cloned osteoblast-like MC3T3-E1 cells, which have been established from neonatal mouse calvaria [Citation21], were maintained as previously described [Citation22]. Briefly, the cells were cultured in 10% fetal bovine serum (FBS)-containing α-minimum essential medium (α-MEM) at 37°C under humidified atmosphere of 5% CO2/95% air [Citation22]. The cells were seeded in 35-mm diameter dishes (5 × 104 cells/dish) or 90-mm diameter dishes (2 × 105 cells/dish) in α-MEM containing 10% FBS [Citation22]. After 5 days, the medium was exchanged for α-MEM containing 0.3% FBS [Citation22]. The cells were used in the experiments after 48 h [Citation22].
Cell migration assay
A transwell cell migration assay was performed according to the method developed by Karagiosis [Citation23] using a Boyden chamber (polycarbonate membrane with 8-µm pores; Transwell®; Corning Costar Corp, Cambridge, MA, USA) as previously described [Citation19,Citation23]. In brief, the cultured MC3T3-E1 cells were seeded (10 × 104 cells/well) onto the upper chamber in α-MEM containing 0.3% FBS [Citation20]. The cells were pretreated with various concentrations of resveratrol or SRT1720 for 60 min in the upper chamber [Citation20], and 10 nM IGF-I was then added to the lower chamber in α-MEM containing 0.3% FBS, and the cells were incubated for 16 h at 37°C [Citation16]. In the case of inauhzin, the cells were preincubated with 1 µM of inauhzin for 60 min in the upper chamber prior to resveratrol treatment. The cells on the upper surface of the membrane were then mechanically removed [Citation20]. The migrated cells adherent to the underside of the membrane were fixed with 4% paraformaldehyde and stained with 4ʹ,6-diamidino-2-phenylindole (DAPI) solution for visualizing nuclei [Citation20]. The migrated cells were photographed and counted using fluorescent microscopy at a magnification of 20× by counting the stained cells from three randomly chosen high-power fields [Citation20].
For wound healing assay, the cultured MC3T3-E1 cells were seeded at 10 × 104 cells/well into an Ibidi Culture-Insert 2 Well (Ibidi, Martinsried, Germany) with a 500-µm margin from the side of the well and allowed to grow for 24 h [Citation16]. After the insert was removed, the cells were pretreated with 10 µM of resveratrol or 3 µM of SRT1720 for 60 min [Citation19], and then stimulated by 70 nM of IGF-I for 8 h [Citation16]. In the case of PD98059, the cells were preincubated with 50 µM of PD98059 for 60 min prior to resveratrol treatment. The cells were photographed using an EOS Kiss X4 digital camera (Canon, Tokyo, Japan) connected to CK40 culture microscope (Olympus Optical Co. Ltd., Tokyo, Japan) before the stimulation of IGF-I and after 8 h [Citation16]. The area of migrated cells was measured by ImageJ software (version 1.48; National Institute of Health, Bethesda, MD, USA) [Citation16]. Regarding the concentration of SRT1720, we previously investigated the effects of various doses of SRT1720 on the migration of MC3T3-E1 cells induced by EGF in Boyden chamber assay, and the maximum effect on migration was observed with 3 µM of SRT1720 [Citation20]. Thus, we adopted 3 µM of SRT1720 for wound healing assay. Regarding the concentration of IGF-I, we previously used 10 nM IGF-I for Boyden chamber assay and 70 nM IGF-I for wound healing assay [Citation24]. Thus, we adopted the same concentration of IGF-I for Boyden chamber assay and wound healing assay in this study.
Western blot analysis
The cultured cells were pretreated with various doses of resveratrol or SRT1720 for 60 min, and then stimulated by 10 nM of IGF-I or vehicle in 1 mL of α-MEM containing 0.3% FBS for the indicated periods [Citation20]. The cells were then lysed, homogenized, and sonicated in a lysis buffer containing 62.5 mM Tris/HCl, pH 6.8, 2% sodium dodecyl sulfate (SDS), 50 mM dithiothreitol, and 10% glycerol [Citation20]. SDS polyacrylamide gel electrophoresis (PAGE) was performed using the method of Laemmli [Citation25] in 10% polyacrylamide gels [Citation20]. The protein was fractionated and transferred onto an Immun-Blot polyvinylidine difluoride membrane (Bio-Rad, Hercules, CA, USA). The membranes were blocked with 5% fat-free dry milk in tris-buffered saline-Tween (TBS-T; 20 mM Tris/HCl, pH 7.6, 137 mM NaCl, 0.1% Tween 20) for 1 h before incubation with primary antibodies [Citation20]. A Western blot analysis was performed as described previously [Citation26] using antibodies against phospho-specific p44/p42 MAP kinase, p44/p42 MAP kinase, phospho-specific Akt, and Akt as primary antibodies with peroxidase-labeled antibodies raised in goat against rabbit IgG (KPL, Inc., Gaitherburg, MD, USA) being used as secondary antibodies [Citation16]. The primary and secondary antibodies were diluted to optimal concentrations with 5% fat-free dry milk in TBS-T [Citation16]. The peroxidase activity on the membrane was visualized on X-ray film using an ECL Western blotting detection system [Citation16]. Regarding the concentration of SRT1720, we reported that 20 µM of SRT1720 significantly suppressed the phosphorylation of p44/p42 MAP kinase induced by prostaglandin F2α in MC3T3-E1 cells [Citation27]. Therefore, we herein adopted the condition (30 µM of SRT1720) similar to the previous study for the signaling experiments in this study. On the other hand, we previously used 10 nM IGF-I for Western blotting [Citation24]. Thus, we adopted the same concentration of IGF-I in this study.
Densitometric analysis
A densitometric analysis of the Western blots was carried out using a scanner and ImageJ software [Citation20]. The phosphorylated levels were calculated as follows: the background-subtracted signal intensity of each phosphorylation signal was normalized to the respective intensity of total protein, and plotted as the fold increase in comparison to that of the control cells without stimulation [Citation20].
Statistical analysis
The data were analyzed by an analysis of variance (ANOVA) followed by Bonferroni method for multiple comparisons between pairs, and p < 0.05 was considered to be statistically significant [Citation20]. All data are presented as the mean ± standard error of the mean (SEM) of triplicate determinations from three independent cell preparations [Citation20].
Results
Effect of resveratrol on the IGF-I-induced migration of MC3T3-E1 cells
We first examined the effect of resveratrol on the IGF-I-induced migration of osteoblast-like MC3T3-E1 cells using a Boyden chamber. Resveratrol, which had little effect on the migration when administered alone, significantly suppressed the IGF-I (10 nM)-induced osteoblast migration in a dose-dependent manner within the range of 0.1 and 10 µM. Resveratrol (10 µM) caused an approximately 50% decrease in the number of migrated cells/area compared to the value with IGF-I alone (). We next examined the effect of resveratrol on the IGF-induced migration of MC3T3-E1 cells using a wound healing assay. The wound healed area after the treatment with IGF-I (70 nM) was significantly reduced by resveratrol (10 µM) (). The effect of resveratrol (10 µM) showed approximately 30% decrease in the effect of IGF-I.
Figure 1. Effect of resveratrol on the IGF-I-induced migration of MC3T3-E1 cells The migration was evaluated using a Boyden chamber. The cells were pretreated with various concentrations of resveratrol for 60 min, and then stimulated by 10 nM of IGF-I or vehicle for 16 h. Representative photographs from triplicate independent experiments, and a histogram showing the number of migrated cells in each panel are presented. The blue spots indicate the nuclei of migrated osteoblasts stained by DAPI. *p < 0.05 compared to the value of the control cells without IGF-I stimulation. **p < 0.05 compared to the value of IGF-I alone

Figure 2. Effect of resveratrol on the IGF-I-induced migration of MC3T3-E1 cells

Effect of SRT1720 on the IGF-I-induced migration of MC3T3-E1 cells
Most of the anti-apoptotic or anti–inflammatory effects of resveratrol are related to the activation of SIRT1, a nicotinamide adenine dinucleotide dependent deacetylase that activates genes associated with survival and longevity [Citation18,Citation19]. Therefore, to confirm that the suppressive effect of resveratrol on the IGF-I-induced osteoblast migration is mediated through SIRT1 activation, we investigated the effect of SRT1720, a direct activator of SIRT1 [Citation28], on the IGF-I-induced migration of osteoblast-like MC3T3-E1 cells. Transwell cell migration assay showed that SRT1720 significantly suppressed the IGF-I (10 nM)-induced osteoblast migration in a dose-dependent manner within the range of 0.1 and 3 µM (). SRT1720 (3 µM) caused an approximately 60% decrease in the number of migrated cells/area compared to the value with IGF-I alone. In addition, wound healing assay showed that SRT1720 reduced the IGF-I (70 nM)-induced osteoblast migration (). The effect of SRT1720 (3 µM) showed approximately 40% decrease in the IGF-I effect.
Figure 3. Effect of SRT1720 on the IGF-I-induced migration of MC3T3-E1 cells

Figure 4. Effect of SRT1720 on the IGF-I-induced migration of MC3T3-E1 cells

Effect of inauhzin, a SIRT1 inhibitor, on the suppression by resveratrol of IGF-I-induced migration of MC3T3-E1 cells
To further clarify whether the suppressive effect on the IGF-I-induced migration of MC3T3-E1 cells is mediated through SIRT1, we performed an additional experiment using inauhzin, a SIRT1 inhibitor [Citation29], and investigated whether SIRT1 activation was truly involved in the suppression of IGF-I-induced migration of MC3T3-E1 cells by resveratrol in Boyden chamber assay. Inauhzin significantly rescued the inhibitory effect by resveratrol on IGF-I-induced cell migration ().
Figure 5. Effect of inauhzin on the suppression by resveratrol of IGF-I-induced phosphorylation of p44/p42 MAP kinase in MC3T3-E1 cells

Effect of resveratrol on the IGF-I-induced phosphorylation of p44/p42 MAP kinase or Akt in MC3T3-E1 cells
We have previously showed that IGF-I-induced osteoblast migration is mediated through the activation of both p44/p42 MAP kinase and PI3-kinase/Akt pathway in MC3T3-E1 [Citation16]. Therefore, to clarify the mechanism underlying the suppression of IGF-I-induced migration by resveratrol, we investigated the effect of resveratrol on the phosphorylation of p44/p42 MAP kinase and Akt stimulated by IGF-I in these cells. Resveratrol, which alone had little effect on the phosphorylation of p44/p42 MAP kinase, significantly inhibited the IGF-I-stimulated phosphorylation of p44/p42 MAP kinase dose-dependently in the range of 10 and 50 µM (). Resveratrol caused about 60% decrease in the IGF-I effect. However, resveratrol hardly affects the phosphorylation of Akt induced by IGF-I in these cells ().
Figure 6. Effect of resveratrol on the IGF-I-induced phosphorylation of p44/p42 MAP kinase in MC3T3-E1 cells

Figure 7. Effect of resveratrol on the IGF-I-induced phosphorylation of Akt in MC3T3-E1 cells

Effect of SRT1720 on the IGF-I-induced phosphorylation of p44/p42 MAP kinase in MC3T3-E1 cells
Furthermore, we examined the effect of SRT1720 on the IGF-I-induced phosphorylation of p44/p42 MAP kinase in MC3T3-E1 cells. We proved that SRT1720, which did not affect the phosphorylation of p44/p42 MAP kinase, significantly decreased the IGF-I-stimulated phosphorylation of p44/p42 MAP kinase (). SRT1720 caused about 60% reduction in the IGF-I effect.
Figure 8. Effect of SRT1720 on the IGF-I-induced phosphorylation of p44/p42 MAP kinase in MC3T3-E1 cells

Effect of PD98059 on the suppression by resveratrol of IGF-I-induced migration of MC3T3-E1 cells
To strengthen the conclusion that suppression of p44/p42 MAP kinase activity by resveratrol is specific to IGF-I-induced osteoblast migration, we further investigated the effect of PD98059, which is known as a specific inhibitor for the upstream kinase of p44/p42 MAP kinase [Citation30], on the IGF-I-induced migration of MC3T3-E1 cells. PD98059 alone suppressed the IGF-I-induced migration of osteoblast, and the suppressive effect of PD98059 is similar to the effect of resveratrol. However, PD98059 did not affect the suppressive effect of resveratrol on osteoblast migration induced by IGF-I, and showed no synergistic effect with resveratrol ().
Figure 9. Effect of PD98059 on the suppression by resveratrol of IGF-I-induced migration of MC3T3-E1 cells

Discussion
In the present study, we showed that resveratrol, a natural polyphenol abundantly found in grapes, berries and red wine, suppressed the migration of osteoblast-like MC3T3-E1 cells induced by IGF-I evaluated with Boyden chamber transwell migration assay and wound healing assay. It has been known that the favorable effects on human health due to the anti–inflammatory and antioxidant properties of resveratrol are mainly exerted by the activation of SIRT1 [Citation19], a gene associated with cell survival and longevity [Citation18]. Thus, we examined the effects of SRT1720, which activates SIRT1 with 1000-fold higher potency than resveratrol [Citation28], on the IGF-I-induced migration of MC3T3-E1 cells, and found that SRT1720 also suppressed the migration similar to resveratrol. In addition, inauhzin, a SIRT1 inhibitor, significantly rescued the inhibitory effect of resveratrol on IGF-I-induced cell migration. Therefore, it is likely that the suppressive effects of resveratrol on the IGF-I-induced osteoblast migration is exerted at least partly through the activation of SIRT1. However, there is not much difference between the inhibitory effect of resveratrol and that of SRT1720 in terms of cell migration. It has been shown that SRT1720 is considered to be 1000 times more potent than resveratrol in activating SIRT1 [Citation28]. However, this effect has been shown to depend on the concentration of resveratrol and SRT1720. In this study, we performed cell migration experiments using resveratrol at a concentration of 0.1–10 µM and SRT1720 at a concentration of 0.1–3 µM, and observed no significant difference in cell migration within these concentrations.
Regarding the intracellular signaling of IGF-I in osteoblasts, we have previously reported that IGF-I stimulates the activation of p44/p42 MAP kinase and Akt in osteoblast-like MC3T3-E1 cells, resulting in the upregulation of ALP activity [Citation13,Citation14]. Based on the previous findings, we investigated the effects of resveratrol on the IGF-I-induced phosphorylation of p44/p42 MAP kinase and Akt in these cells. As a result, resveratrol markedly decreased the phosphorylation of p44/p42 MAP kinase induced by IGF-I, but failed to affect the phosphorylation of Akt. In addition, SRT1720 remarkably decreased the phosphorylation of p44/p42 MAP kinase induced by IGF-I. Therefore, it is most probable that the suppressive effect of resveratrol on phosphorylation of p44/p42 MAP kinase is mediated by SIRT1 activation. Furthermore, PD98059, an inhibitor for p44/p42 MAP kinase [Citation30], alone suppressed the migration of osteoblast induced by IGF-I, but did not affect the suppressive effect of resveratrol on osteoblast migration induced by IGF-I. Therefore, it is likely that resveratrol suppresses IGF-I-induced osteoblast migration through the attenuation of p44/p42 MAP kinase but not Akt, and that SIRT1 activation is involved in the effect of resveratrol at least in part. As for the resveratrol effect on the migration of osteoblasts, we have previously showed that resveratrol suppresses the EGF-induced migration of MC3T3-E1 cells via SIRT1-dependent fashion [Citation20]. However, neither resveratrol nor SRT1720 hardly affected EGF-induced phosphorylation of p44/p42 MAP kinase, whereas both of them could suppress the EGF-stimulated phosphorylation of Akt in MC3T3-E1 cells [Citation20]. Thus, the signaling mechanism underlying the inhibitory effect of resveratrol on the migration might be quite different in each stimulation to osteoblasts. As a whole, from the present findings, it is most likely that resveratrol affects a point upstream of p44/p42 MAP kinase to suppress the IGF-I-induced migration of osteoblasts. The potential mechanism is summarized in .
Figure 10. Schematic illustration of the mechanism underlying the effects of resveratrol on the IGF-I-induced migration of osteoblast-like MC3T3-E1cells

The migration of osteoblasts is a crucial step in bone formation [Citation15]. Generally, the bone remodeling process is initiated with bone resorption by osteoclasts [Citation7]. Subsequently, osteoblasts migrate to the bone resorption sites for the bone formation [Citation15,Citation31]. To maintain the quantity and quality of bone mass, appropriate levels of osteoblast migration are essential for regulating physiological bone remodeling [Citation15]. In addition to the physiological effect, osteoblast migration is also involved in pathological bone diseases, such as fracture repair distress and osteoporosis [Citation15,Citation32]. In the pathological conditions, it is well established that bone turn-over is increased and that bone resorption exceeds bone formation [Citation33]. IGF-I, which plays an essential role in the regulation of bone remodeling process is recognized to be embedded into the bone matrix [Citation9,Citation10]. Thus, IGF-I release could be upregulated in the bone resorption site, leading to excess osteoblast migration causing dislocation of functional osteoblast. Taking into account the remarkable findings shown here, resveratrol could possibly regulate bone remodeling to the appropriate levels for the renewal of bone through the suppression of osteoblast migration, which might be related to the favorable effect of red wine on bone health amelioration [Citation6]. Further investigation is necessary to clarify the exact mechanism by which resveratrol affects osteoblast migration and bone metabolism.
In conclusion, our results strongly suggest that resveratrol suppresses IGF-I-induced osteoblast migration through the attenuation of the p44/p42 MAP kinase pathway and that the effect is mediated at least partly via SIRT1 activation
Authors’ contribution
T.H., H.T., H.I., and O.K. conceived and designed the experiments. T.H., T.K., G.S., K.F., G.K., T.D., W.K., and R.M-N. performed the experiments. T.H., H.T., R.M-N., W.K., and O.K. analyzed the data. T.H., H.T., G.K., H.I., and O.K. wrote the paper. All authors read and approved the final manuscript.
Acknowledgments
We are very grateful to Mrs. Yumiko Kurokawa for her skillful technical assistance. This study was supported in part by Grant-in-Aid for Scientific Research (15K10487 and 17K11002) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and the Research Funding for Longevity Sciences (28-9 and 29-12) from National Center for Geriatrics and Gerontology, Japan.
Disclosure statement
All authors declare that they have no conflicts of interest in connection with this paper.
Additional information
Funding
References
- Rauf A, Imran M, Suleria HAR, et al. A comprehensive review of the health perspectives of resveratrol. Food Funct. 2017;8(12):4284–4305.
- Yap S, Qin C, Woodman OL. Effects of resveratrol and flavonols on cardiovascular function: physiological mechanisms. Biofactors. 2010;36(5):350–359.
- Sawda C, Moussa C, Turner RS. Resveratrol for Alzheimer’s disease. Ann N Y Acad Sci. 2017;1403(1):142–149.
- Renaud S, de Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet. 1992;339(8808):1523–1526.
- Biagi M, Bertelli AA. Wine, alcohol and pills: what future for the French paradox? Life Sci. 2015;131:19–22.
- Kubo JT, Stefanick ML, Robbins J, et al. Preference for wine is associated with lower hip fracture incidence in post menopausal women. BMC Womens Health. 2013;13(1):36.
- Karsenty G, Wagner EF. Reaching a genetic and molecular understanding of skeletal development. Dev Cell. 2002;2(4):389–406.
- Sims NA, Gooi JH. Bone remodeling: multiple cellular interactions required for coupling of bone formation and resorption. Semin Cell Dev Biol. 2008;19(5):444–451.
- Clements TL, Chernausek SD. Genetic strategies for elucidating insulin-like growth factor action in bone. Growth Horm IGF Res. 2004;14(3):195–199.
- Niu T, Rosen CJ. The insulin-like growth factor-1 gene and osteoporosis: A critical appraisal. Gene. 2005;361:38–56.
- Lombardi G, Tauchmanova L, Di Somma C, et al. Somatopause: dismetabolic and bone effects. J Endocrinol Invest. 2005;28(10 Suppl):36–42.
- Garnero P, Sornay-Rendu E, Delmas PD. Low serum IGF-1 and occurrence of 3 osteoporotic fractures in postmenopausal women. Lancet. 2000;355(9207):898–899.
- Noda T, Tokuda H, Yoshida M, et al. Possible involvement of phosphatidylinositol 3-kinase/Akt pathway in insulin-like growth factor-I-induced alkaline phosphatase activity in osteoblasts. Horm Metab Res. 2005;37(5):270–274.
- Hanai Y, Tokuda H, Ishisaki A, et al. Involvement of p44/p42 MAP kinase in insulin-like growth factor-I- induced alkaline phosphatase activity in osteoblast-like MC3T3-E1 cells. Mol Cell Endocrinol. 2006;251(1–2):42–48.
- Su P, Tian Y, Yang C, et al. Mesenchymal stem cell 13 migration during bone formation and bone diseases therapy. Int J Mol Sci. 2018;19(8):E2343. 14
- Kawabata T, Tokuda H, Sakai G, et al. Repression of IGF-I-induced osteoblast migration by (-)17 epigallocatechin gallate through p44/p42 MAP kinase signaling. Biomed Rep. 2018;9(4):318–326.
- Mizutani K, Ikeda K, Kawai Y, et al. Resveratrol stimulates the proliferation and differentiation of osteoblastic MC3T3 E1 cells. Biochem Biophys Res Commun. 1998;253(3):859–863.
- Howitz KT, Bitterman KJ, Cohen HY, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425(6954):191–196.
- Baur JA, Pearson KJ, Price NL, et al. Resveratrol improves health and survival of mice on a high calorie diet. Nature. 2006;444(7117):337–342.
- Kawabata T, Tokuda H, Fujita K, et al. Resveratrol inhibits the epidermal growth factor-induced migration of osteoblasts: the suppression of SAPK/JNK and Akt. Cell Physiol Biochem. 2017;43(3):1025–1036.
- Sudo H, Kodama HA, Amagai Y, et al. In vitro differentiation and calcification in a new clonal osteogenic cell line derived from newborn mouse calvaria. J Cell Biol. 1983;96(1):191–198.
- Kozawa O, Suzuki A, Tokuda H, et al. Prostaglandin F2α stimulates interleukin-6 synthesis via activation of PKC in osteoblast-like cells. Am J Physiol. 1997;272(2 Pt 1):E208–E211.
- Karagiosis SA, Chrisler WB, Bollinger N, et al. Lysophosphatidic acid-induced ERK activation and chemotaxis in MC3T3-E1 preosteoblasts are independent of EGF receptor transactivation. J Cell Physiol. 2009;219(3):716–723.
- Kawabata T, Tokuda H, Sakai G, et al. HSP70 inhibitor suppresses IGF-I-stimulated migration of osteoblasts through p44/p42 MAP Kinase. Biomedicines. 2018;6(4):109.
- Laemmli UK; Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685.
- Kato K, Ito H, Hasegawa K, et al. Modulation of the stress-induced synthesis of hsp27 and αB-crystallin by cyclic AMP in C6 rat glioma cells. J Neurochem. 1996;66(3):946–950.
- Kuroyanagi G, Tokuda H, Matsushima-Nishiwaki R, et al. Resveratrol suppresses prostaglandin F2α-induced osteoprotegerin synthesis in osteoblasts: inhibition of the MAP kinase signaling. Arch Biochem Biophys. 2014;542:39–45.
- Milne JC, Lambert PD, Schenk S, et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450(7170):712–716.
- Zhang Q, Zeng SX, Zhang Y, et al. A small molecule inauhzin inhibits SIRT1 activity and suppresses tumour growth through activation of p53. EMBO Mol Med. 2012;4(4):298‑312.
- Alessi DR, Cuenda A, Cohen P, et al. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270(46):27489–27494.
- Lieberman JR, Daluiski A, Einhorn TA. The role of growth factors in the repair of bone: biology and clinical applications. J Bone Joint Surg Am. 2002;84(6):1032–1044.
- Reddi AH, Roodman D, Freeman C, et al. Mechanisms of tumor metastasis to the bone: challenges and opportunities. J Bone Miner Res. 2003;18(2):190–194.
- Feng X, McDonald JM. Disorders of bone remodeling. Annu Rev Pathol. 2011;6(1):121–145.
