ABSTRACT
Skeletal muscles produce secretory factors termed as myokines, which alter physiological functions of target tissues. We recently identified C-X-C chemokine ligand 10 (CXCL10) as a novel myokine, which is downregulated in response to exercise. In the present study, we investigated whether the nutritional changes affect CXCL10 expression in mouse skeletal muscle. Expression of CXCL10 was evaluated in mice fed a normal diet or a high fat diet for 10 weeks. In animals fed on HFD, Cxcl10 expression was significantly induced in fast-twitched muscles, and was accompanied by increased blood glucose and free fatty acid levels. In vitro experiments using C2C12 myotubes suggested that the increased levels of glucose and palmitic acids directly enhanced CXCL10 expression. Interestingly, the effect of palmitic acids was attenuated by palmitoleic acids. Considering its potent angiostatic activity, induction of CXCL10 by nutritional changes may contribute to the impairment of microvascular networks in skeletal muscles.
GRAPHICAL ABSTRACT
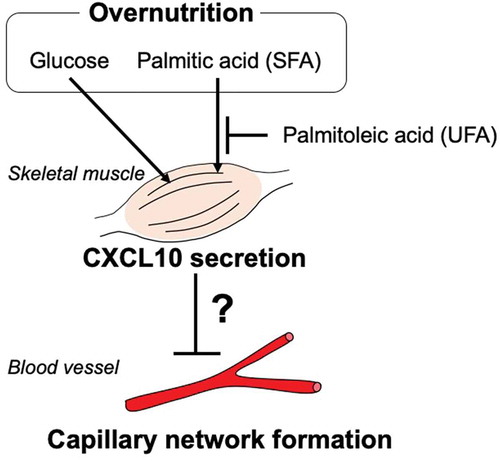
Increased levels of glucose and palmitic acids directly enhanced CXCL10 secretion in skeletal muscles, which may regulate blood vessel formation.
The skeletal muscle has been recognized as an endocrine organ that secretes several types of proteins and peptides, defined as myokines. Notably, the expression of several myokines associated with physical exercise has given rise to the attractive hypothesis that “exercise signals”, which are generated in skeletal muscle, could be transmitted to the whole body via changes in myokine secretion [Citation1]. However, the physiology of skeletal muscle is modulated not only by exercise, but also several other stimuli. It is therefore important to elucidate whether other factors influence the expression and secretion of exercise-regulated myokines.
Currently, the majority of exercise-regulated myokines are up-regulated by physical exercise; however, recent studies demonstrate that some myokines are instead down-regulated by exercise [Citation2]. We recently established a model wherein we applied an electrical pulse stimulation (EPS) to C2C12 myotubes to induce muscle contraction (C2C12-EPS model) [Citation3,Citation4] and identified C-X-C motif chemokine ligand 10 (CXCL10) as a novel exercise-reduced myokine [Citation5].
CXCL10, also known as interferon gamma-induced protein (IP-10), is a multifunctional secreted factor that regulates several biological processes, such as chemotaxis, cell proliferation, and apoptosis [Citation6]. CXCL10 promotes chemotactic activities on effector T cells and other leukocytes [Citation7,Citation8]. However, CXCL10 is also a potent angiostatic chemokine [Citation9]. CXCL10 exerts anti-proliferative effects on endothelial cells [Citation10] and impairs neovascularization in tumors and wound healing [Citation11–13]. The angiostatic activity of CXCL10 can be partially mediated by its high-affinity seven-transmembrane G protein coupled receptor, CXC receptor 3 (CXCR3) expressed in vascular endothelial cells. The activation of CXCR3 induces Ca2+ uptake, followed by activation of the classical caspase-3 cascade and induction of apoptosis [Citation14]. Moreover, Sato et al. reported that CXCL10 antagonizes the functions of basic fibroblasts growth factor (bFGF) in advanced uterine endometrial cancers [Citation15], suggesting that its indirect effect may also contribute to attenuating angiogenesis. Overall, increases in CXCL10 are associated with regressing angiogenesis, which may have physiological and pathological consequences.
Although the regulation of CXCL10 expression has been extensively studied in leukocytes, only a few studies have analyzed its expression in skeletal muscle. Crescioli et al. demonstrated that IFNγ or tumor necrosis factor (TNF)α elicit CXCL10 protein secretion in cultured human skeletal muscle cells [Citation16]. Additionally, we found that the contraction of skeletal muscle is another stimulus that regulates CXCL10 expression [Citation5]. Moreover, peroxisome proliferator-activated receptor (PPAR)γ agonists, such as rosiglitazone or pioglitazone, attenuate the IFNγ-dependent CXCL10 induction in extraocular muscles [Citation17]. Since PPARγ is also expressed in skeletal muscle and activated by exercise, these lines of evidence suggest that exercise-dependent activation of PPARγ may control CXCL10 expression and secretion in skeletal muscle. PPARγ is not only activated by exercise but also by numerous natural compounds. For example, unsaturated fatty acids serve as natural agonists for PPARγ [Citation18]. Given that PPARγ is a nutritional sensor, it could be speculated that nutritional changes may modulate CXCL10 expression and secretion. In fact, Hueso et al. recently demonstrated that plasma levels of CXCL10 are significantly higher in morbidly obese patients than in controls [Citation19]; however, the relationship between nutritional changes and CXCL10 expression is not completely understood.
In this study, we investigated whether high-fat diet-induced obesity affects the expression of CXCL10 in mouse skeletal muscle. Moreover, we utilized an in vitro model to investigate if glucose or free fatty acids (FFA) directly modulate CXCL10 expression in mouse skeletal muscle cells.
Materials and Methods
Animal experiments
All animal care and experiments were approved by the Animal Care Committee at Toyo University. Male C57BL/6 J mice (purchased from Charles River Laboratories Japan Inc., Kanagawa, Japan), 5 weeks of age at the beginning of the experiments, were individually housed in temperature-controlled rooms. The mice were fed either normal diet (Labo MR stock, Nosan Corp., Yokohama, Japan) or high-fat diet (HFD-60; Oriental Yeast Co., Ltd., Tokyo, Japan) and water ad libitum, and were acclimatized to a 12-h light cycle (lights on between 0600 and 1800 h). The mice were weighed every three days. After 10 weeks, mice were anesthetized, and heart blood samples were collected. Then, mice were immediately euthanized by decapitation, and tibialis anterior (TA), extensor digitorum longus (EDL), quadriceps (Quad), and soleus (SOL) skeletal muscles were removed from each hindleg and kept at −80°C until use. Blood glucose levels were measured using Antsense III (Fukuda Denshi, Tokyo, Japan). Serum Non-esterified Fatty Acid (NEFA) levels were measured using LabAssay™ NEFA (FUJIFILM Wako Pure Chemical Corp., Osaka, Japan) according to the manufacturer’s protocol.
Cell culture
Mouse skeletal muscle cell line C2C12 was maintained in DMEM containing 10% fetal bovine serum (FBS), 30 μg/mL penicillin, and 100 μg/mL streptomycin at 37 °C and 5% CO2 atmosphere. The medium was changed every 72 h. For all experiments, cells were grown in 8-well plates (Thermo Fisher Scientific Inc., Waltham, MA, USA) at a density of 5 × 104 cells/well in 2 mL growth medium, or in 6-well plates (Corning Inc., Corning, NY, USA) at a density of 5 × 104 cells/well in 2 mL growth medium. Three days after plating, cells usually reached 100% confluence; their differentiation was subsequently induced by switching to a differentiation medium (DMEM supplemented with 2% calf serum (CS), 30 μg/mL penicillin, and 100 μg/mL streptomycin). The medium was changed every 24 h. After differentiated, C2C12 myotubes were treated with differentiated medium either glucose free (GF, 0.0 g/L glucose), or low glucose (LG, 1.0 g/L glucose), or high glucose (HG, 4.5 g/L glucose) for 24 h. In addition, C2C12 myotubes were also treated with the medium (DMEM supplemented with 2% Albumin, Bovine serum, Fatty Acid Free, pH7.0 (Nacalai tesque, Kyoto, Japan), 30 μg/mL penicillin, and 100 μg/mL streptomycin) that containing palmitic acid (C16:0, 0.5–1.0 mM), or palmitoleic acid (C16:1, 0.5–1.0 mM), or palmitic acid and palmitoleic acid (Mix, C16:0, C16:1, 1 mM) for 24 h.
Western blotting
Proteins were analyzed by western blot as described previously [Citation5]. The cell lysates were prepared using lysis buffer [2% sodium dodecyl sulfate (SDS), 1% 2-mercaptoethanol, 10% glycerol, 0.0033% bromophenol blue, and 50 mM Tris–Cl (pH 6.8)]. The cell lysates were resolved using 10% SDS-polyacrylamide gel electrophoresis (1:30, bis:acrylamide), and transferred to a polyvinylidene difluoride (PVDF) membrane (Immobilon-P; Merck Millipore, Burlington, MA, USA). The membranes were blocked by incubation with 3% bovine serum albumin (BSA) in TBS-T for 30 min. Proteins were detected by 12 h incubation at 4°C with primary anti-phospho AMPK (Thr172, ♯2535S), anti-AMPK (♯2603S) (dilution 1:1000; Cell Signaling Technology, Danvers, MA, USA), and anti-β-actin antibodies (♯AM1021B) (dilution 1;1000, Abcepta, Inc, San Diego, CA, USA). Bands were visualized after subsequent incubation with HRP-conjugated anti-mouse or rabbit IgG (Cell Signaling Technology) and using an ECL detection kit (GE Healthcare Inc.). The intensity of protein bands was quantified using Image J software (http://imagej.nih.gov/ij/).
Quantitative PCR analysis
Fully differentiated C2C12 cells were cultured in the presence of different concentrations of glucose, saturated, or unsaturated fatty acids for 0–24 h. Total RNA was isolated from the cells using TRIzolTM Reagent (Thermo Fisher Scientific Inc.) according to the manufacturer’s protocol. cDNAs were synthesized from 500 ng of total RNA using the PrimeScript RT Master Mix (Takara Bio Inc., Shiga, Japan). Fluorescence real-time PCR analysis was performed using a StepOne instrument (Life Technologies Corporation; Grand Island, NY, USA) and a SYBR Green detection kit (KAPA Biosystems Inc., Woburn, MA, USA) according to the manufacturer’s protocol. The PCR primers for each gene were: mouse Cxcl10, 5ʹ- TCC CTC TCG CAA GGA C − 3ʹ and 5ʹ- TTG GCT AAA CGC TTT CAT −3ʹ; mouse Cox2, 5ʹ-AGA TCA TAA GCG AGG ACC TG-3ʹ and 5ʹ-TAC ACC TCT CCA CCA ATG AC-3ʹ; and mouse Gapdh, 5ʹ- TGT GTC CGT CGT GGA TCT GA −3ʹ and 5ʹ- CGT GCT TCA CCA CCT TCT TGA −3ʹ.
ELISA
CXCL10 concentration in C2C12 culture supernatants or mouse serum was measured with commercially available ELISA kits in accordance with the manufacturer’s protocol (R&D Systems Inc., Minneapolis, MN, USA).
Cell Death Measurement
Cytotoxicity was evaluated using the lactate dehydrogenase (LDH) Cytotoxicity Detection KitPLUS (Roche Diagnostics K.K., Basel, Switzerland) according to the manufacturer’s protocol.
Statistical analysis
All statistical analyzes were performed with Prism 6 software (GraphPad Software Inc., La Jolla, CA, USA). Results are expressed as mean value ± SEM, and the data were analyzed by Student’s t-test or one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test. Differences were considered significant at * p < 0.05, ** p < 0.01, and *** p < 0.001.
Results
HFD enhances Cxcl10 expression in TA and EDL muscle
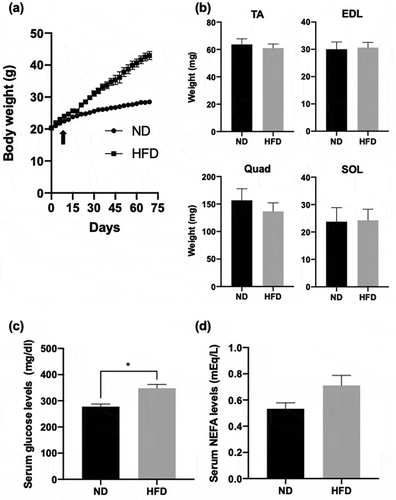
In order to confirm the effects of high-fat diet-induced obesity, 5 week-old male mice were fed either normal diet (ND) or high- fat diet (HFD) for 10 weeks, and their weight was measured every 3 days. As shown in , mice that were exposed to HFD displayed a higher weight gain than those exposed to ND (). After 10 weeks of either ND or HFD, the mice were weighed and then sacrificed; subsequently, the weight of TA, EDL, Quad, and SOL muscles was measured. Although we observed a significant weight gain in HFD group after 10 weeks (mean ± SEM; ND, 26.46 ± 0.45 g; HFD, 34.04 ± 1.08 g; p < 0.001, n = 5) (), the weight of each type of skeletal muscle did not significantly change between the two groups (). Instead, serum glucose levels after 10 weeks were significantly higher in HFD mice than in ND mice (mean ± SEM; ND, 277.5 ± 10.4 mg/dL; HFD, 348.0 ± 14.9 mg/dL; **p < 0.01, n = 5) (). Although the levels of serum NEFA did not significantly differ between ND and HFD mice, they tended to be higher in the HFD group (mean ± SEM; ND, 0.533 ± 0.045 mEq/L; HFD, 0.712 ± 0.076 mEq/L; p = 0.13, n = 5) (). These data suggest that the administration of HFD indeed induced obesity in mice as previously described [Citation20,Citation21].
Figure 1. Effect of HFD on mouse physiology
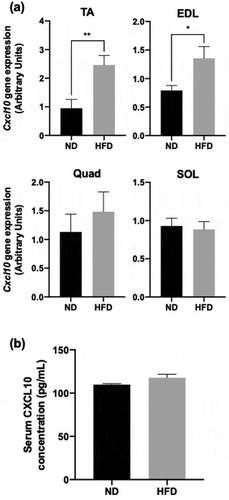
We next investigated the effect of nutritional changes on Cxcl10 expression in each skeletal muscle. As shown , the expression of Cxcl10 in HFD groups was significantly increased in TA and EDL muscles, by approximately 2-fold (), but not affected in Quad or SOL (). As it is well known that TA and EDL muscle predominantly contain fast-twitch muscle [Citation22], these results suggest that HFD particularly affects fast-twitch muscle to increase Cxcl10 expression. Subsequently, we assessed whether nutrition-induced obesity affected blood levels of CXCL10. Surprisingly, only a slight change was observed between the two groups (mean ± SEM; ND, 109.8 ± 1.3 pg/mL; HFD, 117.87 ± 3.89 pg/mL; p = 0.080, n = 8 or 9) ().
Figure 2. Effect of HFD on CXCL10 expression and secretion in mouse skeletal muscles
High glucose enhances CXCL10 production in C2C12 myotubes
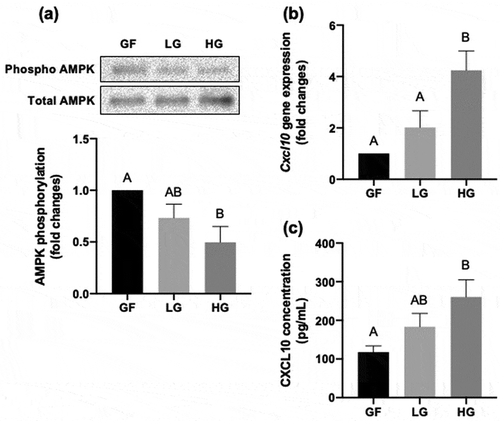
As described, HFD-induced obesity in mice promoted changes in blood nutrition, such as glucose levels (). Next, we utilized C2C12 as a cellular model to elucidate whether glucose or fatty acid administration directly affects the expression of CXCL10. In fully differentiated C2C12 myotubes incubated with DMEM containing different concentrations of glucose for 24 h. Because glucose deprivation modulates phosphorylation of AMP kinase in C2C12 myotubes [Citation23], we re-confirmed that the phosphorylation of AMP kinase was significantly decreased upon increase in glucose levels (). Notably, the expression of Cxcl10 was significantly higher in HG-treated group than in GF or LG-treated groups (). Secretion of CXCL10 was also significantly higher in HG-treated group compared to GF or LG-treated groups (). The HG-dependent CXCL10 induction was not due to cell damage, because no clear relationship was found between cytotoxicity and glucose concentration in culture medium (). Taken together, these data indicate that CXCL10 expression and secretion was enhanced by glucose in C2C12 myotubes.
Figure 3. Effect of glucose on CXCL10 expression and secretion in C2C12 myotubes
Increased levels of saturated fatty acids enhance CXCL10 expression in C2C12 myotubes
Next, we studied the effects of saturated fatty acids (SFA) or unsaturated fatty acids (UFA) on CXCL10 expression. First, we confirmed gene expression of cyclooxygenase-2 (COX2), which was reported to be induced by SFA treatment [Citation24,Citation25]. Differentiated C2C12 myotubes were cultured with or without different concentrations of palmitic acids (C16:0) or palmitoleic acids (C16:1) for 24 h. As shown in , palmitic acid treatment increased the expression of Cox2 in a concentration-dependent manner, a difference which was significant with 1 mM palmitic acid (**p < 0.01, n = 3); instead, palmitoleic acid treatment did not induce significant differences (). Importantly, the induction of Cox2 expression by 1 mM palmitic acid treatment was strongly attenuated in the presence of 1 mM palmitoleic acid (see “mix” in ), which suggests that the composition of SFA and UFA was the crucial factor to determine Cox2 expression.
Figure 4. Effect of palmitic and/or palmitoleic acid on CXCL10 secretion and expression in C2C12 myotubes
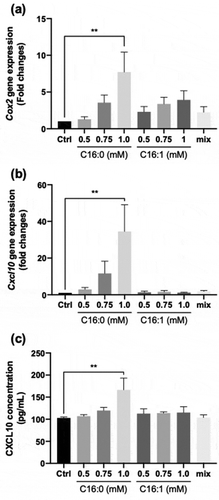
We then evaluated whether CXCL10 gene expression and secretion were altered by treatment with these fatty acids. Similar to Cox2 expression, Cxcl10 expression was strongly and significantly induced by palmitic acid (**p < 0.01, n = 3), but not by palmitoleic acid (). Moreover, the palmitic acid-dependent induction of Cxcl10 expression was diminished by the addition of palmitoleic acid (). Secretion of CXCL10 was also significantly enhanced by palmitic acid treatment, by approximately 1.5-fold, but not by palmitoleic acid (). Again, the palmitic acid-dependent induction of CXCL10 secretion was diminished by the addition of palmitoleic acid (). In any case, no clear relationship was found between cytotoxicity and fatty acid treatments ().
Discussion
Proteins or peptides secreted from skeletal muscle, or myokines, have recently attracted attention since some of them show exercise-regulated expression and secretion [Citation1]. This raises the intriguing possibility that these factors may mediate “exercise signals” from skeletal muscle to other tissues and organs [Citation26–28]. Consequently, numerous exercise-regulated myokines have been identified [Citation4,Citation29,Citation30].
We recently identified CXCL10 as a novel myokine, whose expression is reduced in response to contraction in mouse skeletal muscle [Citation5]. CXCL10 is a potent angiostatic chemokine [Citation9]. Considering that the reduction of capillary density (CD) in skeletal muscle is often associated with numerous diseases, such as diabetes [Citation31,Citation32], cachexia [Citation33], and other chronic diseases [Citation34–36], studies on CD-regulating myokines, including CXCL10, could provide crucial insights to understand these pathological states. To elucidate how the expression of CXCL10 is controlled, we investigated whether nutritional changes affect CXCL10 expression in mouse skeletal muscle.
High-Fat Diet increases Cxcl10 expression in fast-twitch muscles
In HFD-induced obese mice, along with a slight change in serum glucose and free fatty acid levels (), expression of Cxcl10 was significantly increased in TA and EDL muscles, both mainly composed of fast-type fibers (). Although we do not yet have a clear explanation why Cxcl10 expression was increased only in fast-twitched muscles, its induction could be due to the difference in oxidative capacity between fast- and slow-twitch muscles.
Oxidative stress was reported to induce CXCL10 expression in several cell types, including human pancreatic β-cells and human hepatocellular carcinoma cells [Citation37,Citation38]. HFD induces mitochondrial biogenesis, followed by enhanced oxidative capacity in fast-type skeletal muscle, which could be confirmed as fast to slow muscle fiber phenotype changes [Citation39]. In this adaptive process, H2O2 emitting potential of mitochondria increases and redox-buffering capacity decreases [Citation40]. Moreover, Pinho et al. recently suggested that increase in reactive oxygen species (ROS) production and changes in the antioxidant system mainly occur in fast-twitch fibers in HFD-fed rat models [Citation41]. Taking together these reports and our data, it can be speculated that HFD prominently induced oxidative stress in fast-twitch muscles (i.e. TA and EDL muscles), suggesting that induction of Cxcl10 might be fast-type fiber-specific.
Moreover, while Cxcl10 expression in TA and EDL muscles was significantly altered by HFD, serum CXCL10 levels were not substantially affected (). It is indeed possible that the changes in Cxcl10 expression in skeletal muscles were insufficient to influence serum CXCL10 levels; however, Hueso et al. showed that plasma levels of CXCL10 and CXCL11 were significantly higher in morbidly obese patients than in controls [Citation19]. We are thus now investigating whether longer HFD administration, which is sufficient to induce morbid obesity in mouse, may alter blood CXCL10 levels.
It has been reported that the administration high-fat western diet slightly but significantly lowered locomotor activity in mice [Citation42]. Because we previously reported that the forced exercise in mice decreased Cxcl10 expression in skeletal muscles [Citation5], this report raised the possibility that HFD decreased spontaneous physical activity, and this exerts the induction of Cxcl10 expression in skeletal muscles. However, we note that the exercise-dependent reduction of Cxcl10 was predominantly observed in soleus muscle; on the other hand, the significant increases in Cxcl10 expression by HFD was observed in TA and EDL muscles, but not in soleus muscle (). Although further studies are required, these suggest that the regulation of Cxcl10 by physical activity and by nutritional states are distinctly controlled.
Nutritional changes control CXCL10 expression in skeletal muscles
To identify nutritional changes that could be responsible for CXCL10 induction in obese mouse skeletal muscles, we exposed C2C12 cells to increasing amounts of glucose in culture medium and found that higher glucose concentration enhanced CXCL10 secretion. Increased serum CXCL10 levels were reported in human type 1 diabetes [Citation43] and in human type 2 diabetic patients associated with a high glutamic acid decarboxylase antibody (GADA) titer [Citation44]. Although the reason for this increase in serum CXCL10 levels remains elusive, several tissues/cells enhance CXCL10 expression in response to glucose. For example, neonatal hyperglycemia induces Cxcl10 expression in rat hippocampus, which is associated with enhanced oxidative stress [Citation45]. Additionally, CXCL10 is also secreted from monocytes under hyperglycemia [Citation46]. Therefore, our data indicates that hyperglycemia also affects skeletal muscles, inducing increased CXCL10 expression, which may reflect on serum CXCL10 levels.
Interestingly, we found that SFA:UFA ratio is another important factor to determine CXCL10 expression levels in C2C12 myotubes. Numerous reports have evaluated the effects of SFA and/or UFA in skeletal muscles [Citation47,Citation48]. Increased SFA levels contribute to the development of muscle atrophy [Citation49] and insulin resistance [Citation50], effects which are often counteracted by UFA. Although the signaling pathway(s) responsible for SFA-dependent CXCL10 induction remain to be identified, several signaling molecules, such as Forkhead box O 3a (FoxO3a), nuclear factor-κB (NF-κB), protein kinase RNA-like endoplasmic reticulum kinase (PERK), and eukaryotic inducible factor 2 α (eIF2α) seem to be activated by palmitic acid in skeletal muscle cells [Citation51,Citation52]. Moreover, intriguingly, palmitic acid is a potent inducer of ROS in skeletal muscle cells [Citation53], which may support our hypothesis that the overproduction of ROS plays central roles in the induction of CXCL10 in skeletal muscle cells.
Together, we demonstrate that specific nutrients, such as glucose and SFA, directly control CXCL10 expression and secretion in skeletal muscle cells. Nevertheless, it should be noted that other mechanisms may be involved in obesity-dependent Cxcl10 induction in mouse skeletal muscles. Obesity is known to trigger a crosstalk between adipose tissue and skeletal muscle inflammation [Citation54]. Obesity often promotes dysregulated adipokine production and accumulation of pro-inflammatory immune cells, which results in local accumulation of pro-inflammatory cytokines. The abnormal accumulation of pro-inflammatory cytokines then affects skeletal muscle biology, leading to “obese sarcopenia” [Citation54]. Hence, it would also be interesting to investigate the impact of these systems on obesity-dependent CXCL10 induction in skeletal muscle cells.
Conclusion
In this study, we provide evidence that the nutritional state affects the expression of myokine CXCL10 in C2C12 skeletal muscle cells and animal models. Obesity is a risk factor promoting the regression of capillary networks in skeletal muscles, a process in which several factors (i.e. VEGF) are proposed to be involved [Citation55,Citation56]. In addition, it is known that CXCL10 reduces microvessel density in tumor [Citation57]. Together, it is indeed possible to hypothesize that the observed increase in CXCL10 triggered by nutritional changes may also contribute to the impairment of microvascular networks in skeletal muscles, which is one of the potential mechanisms promoting muscle atrophy. To prove this hypothesis, we are currently generating a Cxcl10 conditional knockout mouse model to provide evidence that HFD-dependent Cxcl10 induction directly regulates CD in skeletal muscles.
Authors’ contributions
YI and TN designed the study. YI, EH and AS performed the experiments and analyzed the data. TN wrote the manuscript. All authors have read and approved the final manuscript.
Supplementary_Figure_FV.pdf
Download PDF (87.7 KB)Supplementary material
Supplemental data for this article can be accessed here.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Pedersen BK. Edward F. Adolph Distinguished Lecture: muscle as an endocrine organ: IL-6 and other myokines. J Appl Physiol. 2009;107(4):1006–1014.
- Schnyder S, Handschin C. Skeletal muscle as an endocrine organ: PGC-1α, myokines and exercise. Bone. 2015;80:115–125.
- Fujita H, Nedachi T, Kanzaki M. Accelerated de novo sarcomere assembly by electric pulse stimulation in C2C12 myotubes. Exp Cell Res. 2007;313(9):1853–1865.
- Nedachi T, Fujita H, Kanzaki M. Contractile C 2 C 12 myotube model for studying exercise-inducible responses in skeletal muscle. Am J Physiol Endocrinol Metab. 2008;295(5):E1191–E1204.
- Ishiuchi Y, Sato H, Tsujimura K, et al. Skeletal muscle cell contraction reduces a novel myokine, chemokine (C-X-C motif) ligand 10 (CXCL10): potential roles in exercise-regulated angiogenesis. Biosci Biotech Biochem. 2018;82(1):97–105.
- Sui Y, Potula R, Dhillon N, et al. Neuronal apoptosis is mediated by CXCL10 overexpression in simian human immunodeficiency virus encephalitis. Am J Pathol. 2004;164(5):1557–1566.
- Loetscher M, Gerber B, Loetscher P, et al. Chemokine receptor specific for IP10 and mig: structure, function, and expression in activated T-lymphocytes. J Exp Med. 1996;184(3):963–969.
- Sallusto F, Lenig D, Mackay CR, et al. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998;187(6):875–883.
- Bodnar RJ, Yates CC, Wells A. IP-10 Blocks Vascular Endothelial Growth Factor-Induced Endothelial Cell Motility and Tube Formation via Inhibition of Calpain. Circ Res. 2006;98(5):617–625.
- Luster AD, Greenberg SM, Leder LP. The IP-10 chemokine binds to a specific cell surface heparan sulfate site shared with platelet factor 4 and inhibits endothelial cell proliferation. J Exp Med. 1995;182(1):219–231.
- Luster AD, Cardiff RD, MacLean JA, et al. Delayed wound healing and disorganized neovascularization in transgenic mice expressing the IP-10 chemokine. Proc Assoc Am Physicians. 1998;110(3):183–196.
- Bodnar RJ, Yates CC, Rodgers ME, et al. IP-10 induces dissociation of newly formed blood vessels. J Cell Sci. 2009;122(12):2064–2077.
- Waeckel L, Mallat Z, Potteaux S, et al. Impairment in postischemic neovascularization in mice lacking the CXC chemokine receptor 3. Circ Res. 2005;96(5):576–582.
- Sui Y, Stehno-Bittel L, Li S, et al. CXCL10-induced cell death in neurons: role of calcium dysregulation. Eur J Neurosci. 2006;23(4):957–964.
- Sato E, Fujimoto J, Tamaya T. Expression of interferon-gamma-inducible protein 10 related to angiogenesis in uterine endometrial cancers. Oncology. 2007;73(3–4):246–251.
- Crescioli C, Sottili M, Bonini P, et al. Inflammatory response in human skeletal muscle cells: CXCL10 as a potential therapeutic target. Eur J Cell Biol. 2012;91(2):139–149.
- Antonelli A, Ferrari SM, Corrado A, et al. Extra-ocular muscle cells from patients with Graves‘ ophthalmopathy secrete α (CXCL10) and β (CCL2) chemokines under the influence of cytokines that are modulated by PPARγ. Autoimmune Rev. 2014;13(11):1160–1166.
- Xu HE, Lambert MH, Montana VG, et al. Molecular Recognition of Fatty Acids by Peroxisome Proliferator–Activated Receptors. Mol Cell. 1999;3(3):397–403.
- Hueso L, Ortega R, Selles F, et al. Upregulation of angiostatic chemokines IP-10/CXCL10 and I-TAC/CXCL11 in human obesity and their implication for adipose tissue angiogenesis. Int J Obes (Lond). 2018;42(8):1406–1417.
- Hohos NM, Cho KJ, Swindle DC, et al. High-fat diet exposure, regardless of induction of obesity, is associated with altered expression of genes critical to normal ovulatory function. Mol Cell Endocrinol. 2018;470:199–207.
- Liu P, Lin H, Xu Y, et al. Frataxin-Mediated PINK1-Parkin-Dependent Mitophagy in Hepatic steatosis: the Protective Effects of Quercetin. Mol Nutr Food Res. 2018;62(16):e1800164.
- Augusto V, Padovani CR, Campos GER. Skeletal muscle fiber types in C57BL6J mice. Braz J morphol Sci. 2004;21(2):89–94.
- Ariga M, Yoneyama Y, Fukushima T, et al. Glucose deprivation attenuates sortilin levels in skeletal muscle cells. Endocr J. 2017;64(3):255–268.
- Chen X, Xu S, Wei S, et al. Comparative Proteomic Study of Fatty Acid-treated Myoblasts Reveals Role of Cox-2 in Palmitate-induced Insulin Resistance. Sci Rep. 2016;6(1):21454.
- Coll T, Palomer X, Blanco-Vaca F, et al. Cyclooxygenase 2 Inhibition Exacerbates Palmitate-Induced Inflammation and Insulin Resistance in Skeletal Muscle Cells. Endocrinology. 2010;151(2):537–548.
- Nieto-Vazquez I, Fernàndez-Veledo S, de Alvaro C, et al. Dual role of interleukin-6 in regulating insulin sensitivity in murine skeletal muscle. Diabetes. 2008;57(12):3211–3221.
- Boström P, Wu J, Jedrychowski MP, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481(7382):463–468.
- Peterson JM, Pizza FX. Cytokines derived from cultured skeletal muscle cells after mechanical strain promote neutrophil chemotaxis in vitro. J Appl Physiol (1985). 2009;106(1):130–137.
- Glund S, Deshmukh A, Long YC, et al. Interleukin-6 directly increases glucose metabolism in resting human skeletal muscle. Diabetes. 2007;56(6):1630–1637.
- Høier B, Olsen K, Nyberg M, et al. Contraction-induced secretion of VEGF from skeletal muscle cells is mediated by adenosine. Am J Physiol Heart Circ Physiol. 2010;299(3):857–862.
- Kivelä R, Silvennoinen M, Touvra A-M, et al. Effects of experimental type 1 diabetes and exercise training on angiogenic gene expression and capillarization in skeletal muscle. Faseb J. 2006;20(9):1570–1572.
- Kondo H, Fujino H, Murakami S, et al. Low-intensity running exercise enhances the capillary volume and pro-angiogenic factors in the soleus muscle of type 2 diabetic rats. Muscle Nerve. 2015;51(3):391–399.
- Basic VT, Jacobsen A, Sirsjö A, et al. TNF stimulation induces VHL overexpression and impairs angiogenic potential in skeletal muscle myocytes. Int J Mol Med. 2014;34(1):228–236.
- Kikuchi R, Nakamura K, MacLauchlan S, et al. An antiangiogenic isoform of VEGF-A contributes to impaired vascularization in peripheral artery disease. Nat Med. 2014;20(12):1464–1471.
- Kishlyansky M, Vojnovic J, Roudier E, et al. Striated muscle angio-adaptation requires changes in Vasohibin-1 expression pattern. Biochem Biophys Res Commun. 2010;399(3):359–364.
- Jobin J, Maltais F, Doyon J-F, et al. Chronic obstructive pulmonary disease: capillarity and fiber-type characteristics of skeletal muscle. J Cardiopulm Rehabil. 1998;18(6):432–437.
- Yoshimatsu G, Kunnathodi F, Saravanan PB, et al. Pancreatic β-Cell–Derived IP-10/CXCL10 Isletokine Mediates Early Loss of Graft Function in Islet Cell Transplantation. Diabetes. 2017;66(11):2857–2867.
- You N, Li J, Huang X, et al. COMMD7 activates CXCL10 production by regulating NF-κB and the production of reactive oxygen species.. Mol Med Rep. 2018;17(5):6784–6788.
- Hancock CR, Han D-H, Chen M, et al. High-fat diets cause insulin resistance despite an increase in muscle mitochondria. Proc Natl Acad Sci USA. 2008;105(22):7815–7820.
- Anderson EJ, Lustig ME, Boyle KE, Anderson EJ, Lusting ME, Boyle KE, et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest. 2009;119(3):573–581.
- Pinho RA, Sepa-Kishi DM, Bikopoulos G, et al. High-fat diet induces skeletal muscle oxidative stress in a fiber type-dependent manner in rats. Free Radic Biol Med. 2017;110:381–389.
- Bjursell M, Gerdin A-K, Lelliott CJ, et al. Acutely reduced locomotor activity is a major contributor to Western diet-induced obesity in mice. Am J Physiol Endocrinol Metab. 2008;294(2):E251‐E260.
- Shimada A, Morimoto J, Kodama K, et al. Elevated Serum IP-10 Levels Observed in Type 1 Diabetes. Diabetes Care. 2001;24(3):510–515.
- Suzuki R, Shimada A, Maruyama, et al. T-cell function in anti-GAD65+diabetes with residual β-cell function. J Autoimmun. 2003;20(1):83–90.
- Satrom KM, Ennis K, Sweis BM, et al. Neonatal hyperglycemia induces CXCL10/CXCR3 signaling and microglial activation and impairs long-term synaptogenesis in the hippocampus and alters behavior in rats. J Neuroinflammation. 2018;15(1):82.
- Devaraj S, Jialal I. Increased secretion of IP-10 from monocytes under hyperglycemia is via the TLR2 and TLR4 pathway. Cytokine. 2009;47(1):6–10.
- Rachek LI. Free fatty acids and skeletal muscle insulin resistance. Prog Mol Biol Transl Sci. 2014;121:267–292.
- Dupont J, Dedeyne L, Dalle S, et al. The role of omega-3 in the prevention and treatment of sarcopenia. Aging Clin Exp Res. 2019;31(6):825–836.
- Lipina C, Hundal HS. Lipid modulation of skeletal muscle mass and function. J Cachexia Sarcopenia Muscle. 2017;8(2):190–201.
- Corcoran MP, Lamon-Fava S, Fielding RA. Skeletal muscle lipid deposition and insulin resistance: effect of dietary fatty acids and exercise.. Am J Clin Nutr. 2007;85(3):662–677.
- Woodworth-Hobbs ME, Hudson MB, Rahnert JA, et al. Docosahexaenoic acid prevents palmitate-induced activation of proteolytic systems in C2C12 myotubes. J Nutr Biochem. 2014;25(8):868–874.
- Perry BD, Rahnert JA, Xie Y, et al. Palmitate-induced ER stress and inhibition of protein synthesis in cultured myotubes does not require Toll-like receptor 4. PLoS One. 2018;13(1):e0191313.
- Taheripak G, Bakhtiyari S, Rajabibazl M, et al. Protein tyrosine phosphatase 1B inhibition ameliorates palmitate-induced mitochondrial dysfunction and apoptosis in skeletal muscle cells. Free Radic Biol Med. 2013;65:1435–1446.
- Kalinkovich A, Livshits G. Sarcopenic obesity or obese sarcopenia: A cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res Rev. 2017;35:200–221.
- Gomes JL, Fernandes T, Soci UP, et al. Obesity Downregulates MicroRNA-126 Inducing Capillary Rarefaction in Skeletal Muscle: effects of Aerobic Exercise Training. Oxid Med Cell Longev. 2017;2415246. (Article ID).
- Nwadozi E, Roudier E, Rullman E, et al. Endothelial FoxO proteins impair insulin sensitivity and restrain muscle angiogenesis in response to a high-fat diet. Faseb J. 2016;30(9):3039–3052.
- Yang J, Richmond A. The Angiostatic Activity of Interferon-Inducible Protein-10/CXCL10 In Human Melanoma Depends on Binding to CXCR3 but Not to Glycosaminoglycan. Mol Ther. 2004;9(6):846–855.

