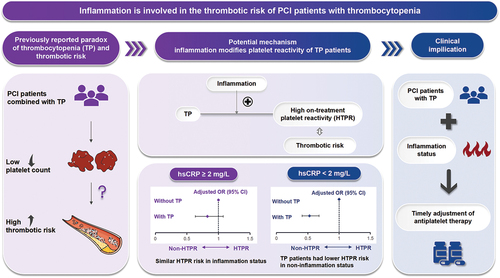
Abstract
Percutaneous coronary intervention (PCI) patients combined with thrombocytopenia (TP) are usually considered to be at low ischemic risk, receiving less proper antiplatelet therapy. However, recent studies reported a paradoxical phenomenon that PCI patients with TP were prone to experience thrombotic events, while the mechanisms and future treatment remain unclear. We aim to investigate whether inflammation modifies platelet reactivity among these patients. Consecutive 10 724 patients undergoing PCI in Fuwai Hospital were enrolled throughout 2013. High-sensitivity C-reactive protein (hsCRP) ≥2 mg/L was considered inflammatory status. TP was defined as platelet count <150×109/L. High on-treatment platelet reactivity (HTPR) was defined as adenosine diphosphate-induced platelet maximum amplitude of thromboelastogram >47mm. Among 6617 patients finally included, 879 (13.3%) presented with TP. Multivariate logistic regression demonstrated that patients with TP were associated with a lower risk of HTPR (odds ratio [OR] 0.64, 95% confidence interval [CI] 0.53–0.76) than those without TP in the overall cohort. In further analysis, among hsCRP <2 mg/L group, patients with TP exhibited a decreased risk of HTPR (OR 0.53, 95% CI 0.41–0.68); however, in hsCRP ≥2mg/L group, TP patients had a similar risk of HTPR as those without TP (OR 0.83, 95% CI 0.63–1.08). Additionally, these results remain consistent across subgroups, including patients presenting with acute coronary syndrome and chronic coronary syndrome. Inflammation modified the platelet reactivity of PCI patients with TP, providing new insights into the mechanisms of the increased thrombotic risk. Future management for this special population should pay more attention to inflammation status and timely adjustment of antiplatelet therapy in TP patients with inflammation.
Plain Language Summary
What is the context?
Recent studies reported a paradoxical phenomenon that percutaneous coronary intervention (PCI) patients with thrombocytopenia (TP) were prone to experience thrombotic events. The potential mechanisms underlying the increased thrombotic risk and how to manage antiplatelet therapy in PCI patients with TP remain unclear.
Growing attention has been paid to immunothrombosis. Inflammation is closely associated with high-on treatment platelet reactivity (HTPR) and thrombotic risk.
HTPR is an independent risk factor of thrombosis and can provide information for guiding antiplatelet therapy.
What is new?
This prospective cohort study enrolled 10 724 patients undergoing PCI in Fuwai Hospital (National Center for Cardiovascular Diseases, Beijing, China), with HTPR risk being the study endpoint of interest.
We first reported that inflammation significantly modified the platelet reactivity of PCI patients with TP.
When hsCRP level <2 mg/L, PCI patients with TP had a decreased risk of HTPR. However, when hsCRP ≥2 mg/L, TP patients had similar HTPR risk as those without TP.
HsCRP levels could modify the relationship between TP and HTPR risks both in patients with acute coronary syndrome and chronic coronary syndrome.
What is the impact?
These results provide insights into potential mechanisms of the increased thrombotic risk in PCI patients with TP. Specifically, inflammation might be involved in the thrombotic risk of PCI patients with TP by modifying the platelet reactivity.
As for future management, personalized antiplatelet therapy should be administrated to TP patients with inflammation status.
Graphical Abstract
Introduction
Platelets play a pivotal role in hemostasis and atherothrombosis.Citation1 Thrombocytopenia (TP), a medical condition characterized by a decreased number of platelets, is relatively common in coronary artery disease (CAD) patients who undergo percutaneous coronary intervention (PCI).Citation2 In general, PCI patients combined with TP are considered to have a lower occurrence of high on-treatment platelet reactivity (HTPR) and a reduced risk of thrombosis, resulting in less probability of being treated with appropriate antiplatelet therapy.Citation3,Citation4 However, recent studies in CAD patients undergoing PCI found that those combined with TP were associated with a higher thrombotic risk despite their low platelet count,Citation5–8 while the underlying mechanisms remain unclear. Clarifying the mechanisms of the increased thrombotic risk in this specific population will provide useful guidance for treatment and further reduce ischemic risk.
Inflammation is closely linked to adverse outcomes.Citation9,Citation10 Previous studies have reported an increased risk of thrombosis in TP patients under inflammatory conditions such as autoimmune diseases and acute infection,Citation11,Citation12 indicating the important role of inflammation in the thrombotic risk of TP patients. As for CAD, a disease often combined with low-grade inflammation, recent experimental studies demonstrated a sophisticated interplay between inflammation and thrombosis in cardiovascular pathology, known as thromboinflammation.Citation13 In addition, our previous studies found that high-sensitivity C-reactive protein (hsCRP), a well-established inflammatory biomarker, was associated with increased risks of HTPR and thrombosis in CAD patients undergoing PCI.Citation14,Citation15 Nonetheless, the mechanisms of the thrombotic risk in PCI patients with TP remain unclear and whether inflammation plays a role in this risk is yet to be elucidated. HTPR is an independent risk factor of thrombosis and can provide information for guiding antiplatelet therapy.Citation16,Citation17 In the present study, we aim to investigate whether inflammation modifies the platelet reactivity of PCI patients with TP, attempting to shed light on the mechanisms of thrombotic risk and generate directions for antiplatelet therapy in this special population.
Methods
Study population
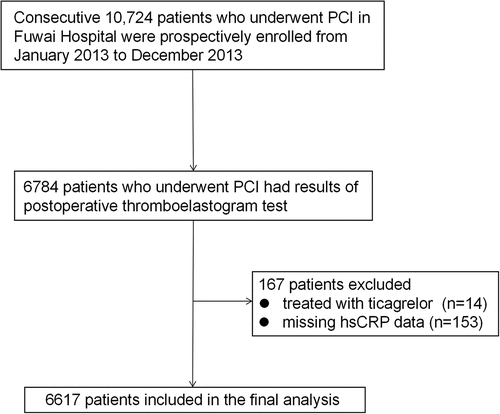
This was a prospective, observational, single-center study that enrolled consecutive 10 724 patients undergoing primary and elective PCI from January 2013 to December 2013 in Fuwai Hospital (National Center for Cardiovascular Diseases, Beijing, China), including patients clinically presented with acute coronary syndrome (ACS) or chronic coronary syndrome (CCS). Among these patients, 6784 had results of postoperative thromboelastogram (TEG) in the real world. After excluding patients who were treated with ticagrelor and with missing data of hsCRP, a total of 6617 patients were included in the final analysis ().
Figure 1. Flowchart of patients enrolled in the study cohort.
PCI was performed by experienced interventional cardiologists blinded to the study protocol. Details on catheterization procedures and periprocedural medication were in line with contemporaneous practice guidelines,Citation18,Citation19 judgment from our team’s experienced cardiologists and their preferences. All subjects received aspirin and clopidogrel before PCI. If patients had not taken antiplatelet drugs before the procedure, they received 300 mg aspirin and 300–600 mg clopidogrel for loading doses. After PCI, patients were prescribed aspirin 100 mg per day indefinitely, and clopidogrel 75 mg per day for at least 1 year. This study protocol was approved by the Fuwai Hospital Ethics Committee, and all patients provided informed consent.
Measurements and definition of inflammation and TP
Within 24 hours after admission, blood samples were obtained after overnight fasting in the morning. We used an automated biochemical analyzer (LABOSPECT 008, HITACHI, Japan) for detecting biochemical indicators, including hsCRP, high-density lipoprotein-cholesterol, low-density lipoprotein-cholesterol, total cholesterol, triglyceride, glucose, and estimated glomerular filtration rate. A Sysmex XN 2000 automated blood cell counter (Sysmex Corporation, Kobe, Japan) was used to measure platelet count, mean platelet volume, and hemoglobin.
Among the numerous inflammatory biomarkers, high-sensitivity C-reactive protein (hsCRP) has the most extensive predictive validation with regard to adverse outcomes,Citation20 and current consensus has defined hsCRP ≥2 mg/L as persistent proinflammatory response and inflammation risk in CAD patients.Citation21,Citation22 In addition, the universal normal range of reference platelet counts was 150–450 ×109/L,Citation23 and studies found that platelet count <150 × 109/L is a relatively common finding in PCI patients.Citation24 Therefore, in the present study, hsCRP ≥2 mg/L was considered as under inflammatory status and TP was defined as platelet counts <150 × 109/L, which also had been adopted in previous studies.
Measurements and definition of HTPR
To assess platelet reactivity, we utilized the TEG@5000 thromboelastograph hemostasis system manufactured by the American Haemoscope Corporation to measure the adenosine diphosphate-induced platelet maximum amplitude [MA(ADP)] parameter, which reflects the maximum amplitude of clots after administering P2Y12 receptor antagonists. During the next morning after PCI, venous blood samples were obtained from patients in a supine position for point-of-care TEG analysis. According to the consensus of the definition of platelet reactivity, HTPR was defined as MA(ADP) >47 mm.Citation25
Statistical analysis
Continuous variables were reported as mean ± standard deviation in normal distribution or median (interquartile range) in the non-normal distribution, while categorical variables were presented as numbers (percentage). Comparisons between continuous variables were performed using the independent sample Student’s t-test or Wilcoxon Mann–Whitney test. Categorical variables were compared using the Pearson chi-square test and Fisher’s exact test. Patients were divided into hsCRP ≥2 mg/L group and hsCRP <2 mg/L group. Univariate logistic regression was used to analyze the relationship between TP and HTPR, both in the entire cohort and in the different hsCRP groups. Significant variables from these univariate analyses were then entered into the corresponding multivariate logistic regression models. The odds ratio (OR) and 95% confidence interval (CI) were calculated. To assess whether inflammation modifies the platelet reactivity of TP patients undergoing PCI with different clinical presentations, subgroup analysis was performed in patients with ACS and CCS, respectively. For sensitivity analysis, we further excluded patients with other causes of systemic inflammation, such as active infection, malignant disease, autoimmune rheumatologic diseases, etc., to rule out potential confounding factors. A two-sided P-value <.05 was considered statistically significant. All analyses in this study were performed using SPSS software version 25.0 (IBM Corp., Armonk, New York, USA).
Results
Baseline characteristics
A total of 6617 eligible patients were ultimately included in this study (). The average age of the participants in this study was 58.25 ± 10.29 years, with 5132 (77.6%) being male, 879 (13.3%) presenting with TP, and 2801 (42.3%) having hsCRP levels ≥2 mg/L. Of the overall population, the mean MA (ADP) of TEG was 35.80 ± 17.65 mm, and 2025 (30.6%) patients had HTPR.
shows the baseline characteristics of patients who are HTPR and those who are non-HTPR. Compared with non-HTPR patients, patients who had HTPR were older, less males, less TP patients and smokers, had a higher prevalence of ACS, diabetes, and hypertension, had lower rates of prior myocardial infarction and prior PCI, had higher levels of hsCRP, low-density lipoprotein-cholesterol, and glucose, had lower levels of hemoglobin and estimated glomerular filtration rate. Additionally, baseline characteristic differences between patients with TP (n = 879) and without TP (n = 5738) are shown in Table S1 and baseline characteristics of hsCRP <2 mg/L group (n = 3816) and hsCRP ≥2 mg/L group (n = 2801) are shown in Table S2.
Table I. Baseline characteristics of patients according to platelet reactivity.
Association between TP and HTPR in the entire cohort
In the entire cohort, the univariate logistic regression model showed that patients with TP had a reduced risk of HTPR than those without TP (OR 0.59, 95% CI 0.50–0.70, p < .001). After including the significant variables in the univariate analysis, multivariate logistic regression analysis still identified that patients with TP had a lower HTPR than patients without TP (adjusted OR 0.64, 95% CI 0.53–0.76, p < .001) (). A significant interaction was observed between TP and hsCRP (p = .005).
Table II. Univariate and multivariate logistic regression for HTPR in entire cohort.
Association between TP and HTPR stratified by hsCRP levels
In the hsCRP <2 mg/L group, the univariate logistic regression model showed that the presence of TP was significantly associated with a reduced risk of HTPR (OR 0.49, 95% CI 0.39–0.63, p < .001) (). After enrolling significant variables in the univariate model, multivariate logistic regression showed that TP patients remained significantly associated with a decreased risk of HTPR than patients without TP (adjusted OR 0.53, 95% CI 0.41–0.68, p < .001) ().
Figure 2. Multivariate logistic regression for HTPR stratified by hsCRP.
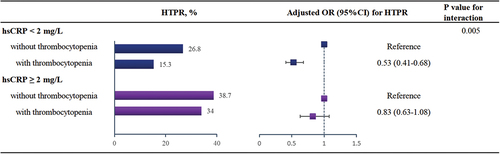
Table III. Univariate logistic regression for HTPR stratified by hsCRP levels.
However, in the hsCRP ≥2 mg/L group, the univariate logistic regression model showed that there was no significant difference in the risk of HTPR between patients with TP and without TP (OR 0.82, 95% CI 0.63–1.05, p = .111) (). Even after adjusting for significant covariates in the univariate model, multivariate logistic regression still showed that the risk of HTPR was similar between patients with TP and without TP (adjusted OR 0.83, 95% CI 0.63–1.08, p = .155) ().
Combined effect of hsCRP and TP for HTPR
Patients who had hsCRP levels ≥2 mg/L and without TP presented the highest proportion of HTPR (38.7%). Both univariate and multivariate logistic regression showed that those concurrently with hsCRP ≥2 mg/L and TP had a similar risk of HTPR with those presenting the highest risk of HTPR (p > .05) ().
Table IV. Combined effect of hsCRP and TP for HTPR.
Subgroup analysis
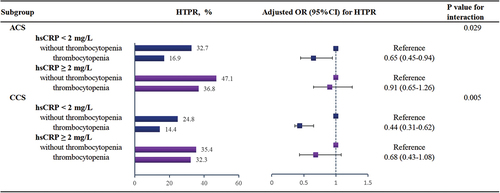
Among patients undergoing PCI, 57.3% (n = 3790) presented with ACS, while 42.7% (n = 2827) had CCS. When compared with those with CCS, ACS patients had a higher proportion of hsCRP ≥2 mg/L (48.1% vs. 34.6%, p < .001), and a higher incidence of HTPR (32.2% vs. 28.5%, p = .001). Multivariate Cox regression analysis demonstrated that in both ACS and CCS subgroups, the relationships between TP and HTPR were all modified by hsCRP levels (both P for interaction < 0.05), which were consistent with the overall population ().
Figure 3. Subgroup analyses of the association between thrombocytopenia and HTPR in different hsCRP levels.
Sensitivity analysis
After further excluding 17 patients with systemic inflammation, including 2 with acute infections, 1 with Hashimoto’s disease, 6 with malignant tumors, and 8 with rheumatologic diseases, a total of 6600 patients were included in the sensitivity analysis. Similarly, we also observed a significant interaction between TP and hsCRP (p = .004). In the hsCRP <2 mg/L group, even after adjusting for significant confounders included in the univariate model (see Supplementary Table S3), the multivariable logistic model showed that TP patients remained significantly associated with a lower risk of HTPR. However, in the hsCRP ≥2 mg/L group, the multivariable logistic regression revealed a similar risk of HTPR between TP and non-TP patients (Figure S1).
Discussion
The present study used a large-sample, real-world, prospective cohort to explore the platelet reactivity in PCI patients with TP across different inflammatory levels. The major findings were as follows. In the overall PCI cohort, patients with TP had a lower risk of HTPR. However, it is worth noting that the association between TP and HTPR was significantly modulated by inflammatory levels. Specifically, when hsCRP <2 mg/L, TP patients still exhibited a lower risk of HTPR than patients without TP; while when hsCRP ≥2 mg/L, patients with TP had a similar risk of HTPR as those without TP. In further analysis of ACS and CCS subgroups, inflammation also notably modulated the platelet reactivity of TP patients. This was the first study to reveal potential differences in the platelet reactivity of PCI patients with TP based on baseline hsCRP levels.
Inflammation, TP, and thrombotic risk
As platelets play a key role in hemostasis and thrombosis, patients with TP are considered to have a higher risk of bleeding and a lower risk of thrombosis. However, it is surprising that current studies about the prognosis of PCI patients with TP yield conflicting data. Yadav et al. and Roh et al., respectively, reported that baseline TP was associated with an increased ischemic risk after PCI in patients with ACS and acute myocardial infarction.Citation6,Citation8 In a large cohort of 65 130 patients who underwent PCI (including patients presented with ACS and CCS), Ayoub et al. revealed that patients with TP were simultaneously at higher risks of in-hospital ischemic and bleeding events.Citation5 Meanwhile, a meta-analysis enrolling 19 studies found a J-shaped relationship between the preprocedural platelet counts and the risk of all-cause mortality and major adverse cardiovascular events following PCI.Citation26 Despite recent studies indicating the inverse relationship between reduced platelet counts and elevated thrombotic risk, the underlying mechanisms contributing to this paradoxical phenomenon remain unclear.
This large-scale real-world study revealed for the first time that when hsCRP level <2 mg/L, a decreased risk of HTPR among PCI patients with TP was observed; however, when hsCRP levels ≥2 mg/L, patients with TP demonstrated a comparable risk of HTPR to those without TP. Previous studies have confirmed HTPR as an independent risk factor for thrombotic events in PCI patients.Citation27–29 Therefore, in light of our findings, we inferred that inflammation might play a role in the mechanism underlying the increased thrombotic risk in PCI patients with TP. It is plausible that inflammation might exert its influence by affecting the platelet reactivity of TP patients and thus increasing their susceptibility to thrombotic risk, providing new insights into the mechanisms.
The adverse impact of inflammation had been demonstrated in TP patients in previous studies and they mainly focused on severe inflammation status. Patients with TP under severe inflammation conditions (such as autoimmune diseases and acute infections) might have an increased risk of thrombosis. For instance, TP patients with autoimmune diseases could exhibit ischemic risk, with incidence rates of thrombosis ranging from 2.2% to 45% in patients with immune thrombocytopenia, 25%–50% in TP patients with systemic lupus erythematosus, and 10%–26% in TP patients with antiphospholipid syndrome.Citation30 Similarly, among patients with COVID-19 infection, approximately one-third of patients presented TP.Citation31 However, the thrombotic events were reported significantly higher than bleeding events in patients with COVID-19 infection.Citation32 Therefore, previous studies consistently confirmed a strong association between severe inflammation status and thrombotic risk in TP patients. Our study mainly focused on CAD patients undergoing PCI who often combined with low-grade inflammation status. We found that even the low-grade inflammation levels in PCI patients could have an impact on TP patients. More importantly, this study revealed that inflammation status could significantly modulate the platelet reactivity of TP patients, which might further contribute to the increased thrombotic risk. In addition, despite the higher inflammation level in ACS patients compared to those with CCS, our results reveal a noteworthy finding. Regardless of whether CAD patients undergoing PCI presented with ACS or CCS, those under inflammatory conditions had a similar risk of HTPR between TP and non-TP individuals. Our results underscore the significant impact of inflammation on platelet reactivity in CAD patients undergoing PCI combined with TP.
Potential basic mechanisms
The present data could be supported by previous experimental observations that under inflammation status, patients with TP might not necessarily exhibit lower platelet reactivity despite their decreased platelet count. TP patients with acute infection or autoimmune diseases could have notable changes such as increased platelet reactivity, platelet volume, elevated platelet particles, and enhanced adhesion to the vascular wall, contributing to a higher thrombotic risk.Citation33,Citation34 Emerging evidence has illustrated that high platelet reactivity could be driven by inflammation in several ways. Recent research established that tumor necrosis factor-α, one of the proinflammatory cytokines, could significantly regulate megakaryocytes in the bone marrow and promote high platelet reactivity.Citation35 Various immune cells such as neutrophils, lymphocytes and monocytes could produce platelet-activating factors, thereby promoting platelet activation and aggregation.Citation36,Citation37 In addition, neutrophil extracellular traps which are released after neutrophil activation can promote platelet activation through histones.Citation38 The mechanistic basis of inflammation enhancing platelet reactivity in TP patients warrants further study.
Clinical implications and future directions
Importantly, our study not only proposed a potential mechanism of the thrombotic risk in PCI patients with TP but also provided a new perspective on clinical decision-making. In this large-sample real-world study, TP prevalence reached 13.3% of PCI patients, higher than that in the general population (3.9%),Citation39 emphasizing the necessity to optimize the personalized management of this relatively common disease in the PCI population. It is noteworthy that patients with TP are frequently withheld from receiving standard antiplatelet therapies due to concerns regarding bleeding.Citation8,Citation40,Citation41 However, recent studies indicated that CAD patients undergoing PCI with concurrent TP were associated with a higher risk of ischemia.Citation6,Citation7 Therefore, it is crucial to timely adjust antiplatelet therapy for TP patients according to thrombotic risk, rather than withholding standard antiplatelet treatments solely for fear of bleeding.
HTPR is an important risk factor for thrombosis and platelet function test is recommended by guidelines to be used as one of references for adjusting antiplatelet strategy.Citation16,Citation17 Our study found that inflammation significantly modulated the risk of HTPR among PCI patients with TP. Therefore, in future clinical practice, special attention should be paid to inflammatory biomarkers in PCI patients with TP, and timely adjustment of antiplatelet regimen could be prescribed to TP patients according to inflammatory status, which applies to PCI patients with TP presenting with clinical manifestations of ACS and CCS. More clinical trials are warranted to explore the optimal antiplatelet therapy regimen for PCI patients with TP when they are under inflammatory status.
Limitation
There were several limitations of this study. First, this was a single-center observational study, which might potentially limit the generalizability of our findings. Secondly, we did not document the proportion of patients who had been receiving clopidogrel before PCI, which may have affected the results.Citation42 Third, this study mainly enrolled patients treated with clopidogrel, and more future studies are needed to explore whether patients using other P2Y12 receptor inhibitors can yield consistent conclusions. Moreover, although relevant variables were included for adjustment as much as possible in this study, there might have other unmeasured confounding factors that affected the association due to limited data, such as inheritance and drug interaction, which may have a certain impact on the results.Citation43,Citation44 Additionally, this study mainly focused on the impact of inflammation on HTPR among TP patients and future research is required to investigate whether the reported results affect long-term outcomes. Lastly, we cannot provide the etiology of thrombocytopenia. However, we can determine that the patients admitted to Fu Wai hospital were mostly related to cardiac diseases and very rarely had comorbid serious or chronic hematologic diseases such as leukemia. Despite these limitations, the large number of patients studied in the real world and novel findings provided valuable data in this special PCI population.
Conclusion
This real-world large-scale cohort study revealed that in the total PCI population, patients with TP had a lower risk of HTPR; however, inflammation could modify the platelet reactivity of patients with TP. These findings not only provided new insights into the potential mechanisms behind the thrombotic risk in PCI patients with TP; but also might have clinical implications on timely adjusting antiplatelet regiments in TP patients with inflammation status.
Abbreviations
| ACS | = | Acute Coronary Syndrome |
| CAD | = | Coronary Artery Disease |
| CCS | = | Chronic Coronary Syndrome |
| CI | = | Confidence Interval |
| hsCRP | = | High-Sensitivity C-Reactive Protein |
| HTPR | = | High-On Treatment Platelet Reactivity |
| MA (ADP) | = | Adenosine Diphosphate Induced Platelet Maximum Amplitude |
| OR | = | Odds Ratio |
| PCI | = | Percutaneous Coronary Intervention |
| TEG | = | Thromboelastogram |
| TP | = | Thrombocytopenia |
Author contributions
YKL, LJW, and ZXY contributed to the concept and design of the study; YKL wrote the manuscript; YKL and LJW conducted the statistical analysis; ZXY and YJQ revised the intellectual content; YKL, LJW, ZP, TXF, LYL, and YDS contributed to data collection; YYJ, and GRL contributed to interpretation of data; all authors approved the final version of the manuscript.
Supplemental Material
Download PDF (363.2 KB)Acknowledgments
We thank all staff contributing to this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Due to ethical restrictions related to the consent given by subjects at the time of study commencement, our datasets are available from the corresponding author upon reasonable request after permission of the Institutional Review Board of State Key Laboratory of Cardiovascular Disease, Fu Wai Hospital, National Center for Cardiovascular Diseases.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/09537104.2024.2327835.
Additional information
Funding
References
- Freynhofer MK, Bruno V, Wojta J, Huber K. The role of platelets in athero-thrombotic events. Curr Pharm Des. 2012;18(33):5197–9. doi:10.2174/138161212803251899.
- Berkowitz SD, Sane DC, Sigmon KN, Shavender JH, Harrington RA, Tcheng JE, Topol EJ, Califf RM. Occurrence and clinical significance of thrombocytopenia in a population undergoing high-risk percutaneous coronary revascularization. Evaluation of c7E3 for the prevention of ischemic complications (EPIC) study group. J Am Coll Cardiol. 1998;32(2):311–9. doi:10.1016/s0735-1097(98)00252-6.
- WANG TY, OU FS, ROE MT, Harrington RA, Ohman EM, Gibler WB, Peterson ED. Incidence and prognostic significance of thrombocytopenia developed during acute coronary syndrome in contemporary clinical practice. Circulation. 2009;119(18):2454–62. doi:10.1161/circulationaha.108.827162.
- Ranucci M, Baryshnikova EFTS, CLinical Outcome Research Score G. The interaction between preoperative platelet count and function and its relationship with postoperative bleeding in cardiac surgery. Platelets. 2017;28(8):794–8. doi:10.1080/09537104.2017.1280148.
- Ayoub K, Marji M, Ogunbayo G, Masri A, Abdel-Latif A, Ziada K, Vallurupalli S. Impact of chronic thrombocytopenia on in-hospital outcomes after percutaneous coronary intervention. JACC Cardiovasc Interv. 2018;11(18):1862–8. doi:10.1016/j.jcin.2018.05.033.
- Yadav M, Genereux P, Giustino G, Madhavan MV, Brener SJ, Mintz G, Caixeta A, Xu K, Mehran R, Stone GW, et al. Effect of baseline thrombocytopenia on ischemic outcomes in patients with acute coronary syndromes who undergo percutaneous coronary intervention. Can J Cardiol. 2016;32(2):226–33. doi:10.1016/j.cjca.2015.05.020.
- Overgaard CB, Ivanov J, Seidelin PH, Todorov M, Mackie K, Džavík V. Thrombocytopenia at baseline is a predictor of inhospital mortality in patients undergoing percutaneous coronary intervention. Am Heart J. 2008;156(1):120–4. doi:10.1016/j.ahj.2008.02.003.
- Roh JW, Lim S, HWang Y, Lee KY, Choo EH, Choi IJ, Hwang B-H, Kim CJ, Park M-W, Kim D-B, et al. Ischemic and bleeding events associated with thrombocytopenia and thrombocytosis after percutaneous coronary intervention in patients with acute myocardial infarction. J Clin Med. 2020;9(10):3370. doi:10.3390/jcm9103370.
- Wada H, Dohi T, Miyauchi K, Shitara J, Endo H, Doi S, Naito R, Konishi H, Tsuboi S, Ogita M, et al. Preprocedural high-sensitivity C-reactive protein predicts long-term outcome of percutaneous coronary intervention. Circ J. 2016;81(1):90–5. doi:10.1253/circj.CJ-16-0790.
- Duewell P, KOno H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nuñez G, Schnurr M, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464(7293):1357–61. doi:10.1038/nature08938.
- ANdic N, Gunduz E, AKay OM, Şahin D, Teke HÜ. Cardiac and pulmonary thrombosis during multidrug treatment in an idiopathic thrombocytopenic purpura patient. Platelets. 2014;25(1):69–70. doi:10.3109/09537104.2012.758360.
- Mcfadyen JD, Stevens H, Peter K. The emerging threat of (micro)thrombosis in COVID-19 and its therapeutic implications. Circ Res. 2020;127(4):571–87. doi:10.1161/circresaha.120.317447.
- Stark K, Massberg S. Interplay between inflammation and thrombosis in cardiovascular pathology. Nat Rev Cardiol. 2021;18(9):666–82. doi:10.1038/s41569-021-00552-1.
- Zhao X, Jiang L, Xu L, Tian J, Xu Y, Zhao Y, Feng X, Wu Y, Zhang Y, Wang D, et al. Predictive value of in-hospital white blood cell count in Chinese patients with triple-vessel coronary disease. Eur J Prev Cardiol. 2019;26(8):872–82. doi:10.1177/2047487319826398.
- Li J, Yuan D, Jiang L, Tang X, Xu J, Song Y, Chen J, Qiao S, Yang Y, Gao R, et al. Similar inflammatory biomarkers reflect different platelet reactivity in percutaneous coronary intervention patients treated with clopidogrel: a large-sample study from China. Front Cardiovasc Med. 2021;8:736466. doi:10.3389/fcvm.2021.736466.
- Collet JP, THiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, Dendale P, Dorobantu M, Edvardsen T, Folliguet T, et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42(14):1289–367. doi:10.1093/eurheartj/ehaa575.
- Neumann FJ, Sousa-uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet J-P, Falk V, Head SJ, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. EuroIntervention. 2019;14(14):1435–534. doi:10.4244/eijy19m01_01.
- Wijns W, Kolh P, Danchin N, Di Mario C, Falk V, Folliguet T, Garg S, Huber K, James S, Knuuti J, et al. Guidelines on myocardial revascularization. Eur Heart J. 2010;31(20):2501–55. doi:10.1093/eurheartj/ehq277.
- Levine GN, BAtes ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention. A report of the American college of cardiology foundation/American heart association task force on practice guidelines and the society for cardiovascular angiography and interventions. J Am Coll Cardiol. 2011;58(24):e44–122. doi:10.1016/j.jacc.2011.08.007.
- RIdker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342(12):836–43. doi:10.1056/nejm200003233421202.
- Ridker PM. Residual inflammatory risk: addressing the obverse side of the atherosclerosis prevention coin. Eur Heart J. 2016;37(22):1720–2. doi:10.1093/eurheartj/ehw024.
- Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–31. doi:10.1056/NEJMoa1707914.
- Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M, Vardiman JW. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–405. doi:10.1182/blood-2016-03-643544.
- Lewis B. Thrombocytopenia and outcome in invasive cardiology. J Invasive Cardiol, 2002;14 Suppl B:38b–47b.
- Tantry US, Bonello L, ARadi D, Price MJ, Jeong Y-H, Angiolillo DJ, Stone GW, Curzen N, Geisler T, ten Berg J, et al. Consensus and update on the definition of on-treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding. J Am Coll Cardiol. 2013;62(24):2261–73. doi:10.1016/j.jacc.2013.07.101.
- Galimzhanov A, Sabitov Y, Tenekecioglu E, Tun HN, Alasnag M, Mamas MA. Baseline platelet count in percutaneous coronary intervention: a dose–response meta-analysis. Heart. 2022;108(22):1792–9. doi:10.1136/heartjnl-2022-320910.
- Montalescot G, Rangé G, Silvain J, Bonnet J-L, Boueri Z, Barthélémy O, Cayla G, Belle L, Van Belle E, Cuisset T, et al. High on-treatment platelet reactivity as a risk factor for secondary prevention after coronary stent revascularization: a landmark analysis of the ARCTIC study. Circulation. 2014;129(21):2136–43. doi:10.1161/circulationaha.113.007524.
- Patti G, NUsca A, Mangiacapra F, Gatto L, D’Ambrosio A, Di Sciascio G. Point-of-care measurement of clopidogrel responsiveness predicts clinical outcome in patients undergoing percutaneous coronary intervention results of the ARMYDA-PRO (antiplatelet therapy for reduction of myocardial damage during angioplasty-platelet reactivity predicts outcome) study. J Am Coll Cardiol. 2008;52(14):1128–33. doi:10.1016/j.jacc.2008.06.038.
- Marcucci R, Gori AM, Paniccia R, Giusti B, Valente S, Giglioli C, Buonamici P, Antoniucci D, Abbate R, Gensini GF, et al. Cardiovascular death and nonfatal myocardial infarction in acute coronary syndrome patients receiving coronary stenting are predicted by residual platelet reactivity to ADP detected by a point-of-care assay: a 12-month follow-up. Circulation. 2009;119(2):237–42. doi:10.1161/circulationaha.108.812636.
- Han X, Li C, Zhang S, Hou X, Chen Z, Zhang J, Zhang Y, Sun J, Wang Y. Why thromboembolism occurs in some patients with thrombocytopenia and treatment strategies. Thromb Res. 2020;196:500–9. doi:10.1016/j.thromres.2020.10.005.
- Guan WJ, Ni ZY, Hu Y, Liang W-H, Ou C-Q, He J-X, Liu L, Shan H, Lei C-L, Hui DSC, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–20. doi:10.1056/NEJMoa2002032.
- Mei H, Luo L, Hu Y. Thrombocytopenia and thrombosis in hospitalized patients with COVID-19. J Hematol Oncol. 2020;13(1):161. doi:10.1186/s13045-020-01003-z.
- Handtke S, Thiele T. Large and small platelets-(when) do they differ? J Thromb Haemost, 2020, 18(6): 1256–67. DOI: 10.1111/jth.14788.
- Boulware R, Refaai MA. Why do patients with immune thrombocytopenia (ITP) experience lower bleeding events despite thrombocytopenia? Thromb Res. 2020;187:154–8. doi:10.1016/j.thromres.2020.01.020.
- Davizon-castillo P, Mcmahon B, Aguila S, Bark D, Ashworth K, Allawzi A, Campbell RA, Montenont E, Nemkov T, D’Alessandro A, et al. TNF-α–driven inflammation and mitochondrial dysfunction define the platelet hyperreactivity of aging. Blood. 2019;134(9):727–40. doi:10.1182/blood.2019000200.
- Demopoulos C, Antonopoulou S, Theoharides TC. COVID-19, microthromboses, inflammation, and platelet activating factor. Biofactors. 2020;46(6):927–33. doi:10.1002/biof.1696.
- Lordan R, Tsoupras A, Zabetakis I, Demopoulos CA. Forty years since the structural elucidation of platelet-activating factor (PAF): historical, Current, and future research perspectives. Molecules. 2019;24(23):4414. doi:10.3390/molecules24234414.
- Semeraro F, Ammollo CT, Morrissey JH, Dale GL, Friese P, Esmon NL, Esmon CT. Extracellular histones promote thrombin generation through platelet-dependent mechanisms: involvement of platelet TLR2 and TLR4. Blood. 2011;118(7):1952–61. doi:10.1182/blood-2011-03-343061.
- Biino G, Balduini CL, Casula L, Cavallo P, Vaccargiu S, Parracciani D, Serra D, Portas L, Murgia F, Pirastu M, et al. Analysis of 12,517 inhabitants of a Sardinian geographic isolate reveals that predispositions to thrombocytopenia and thrombocytosis are inherited traits. Haematologica. 2011;96(1):96–101. doi:10.3324/haematol.2010.029934.
- Hakim DA, Dangas GD, Caixeta A, Nikolsky E, Lansky AJ, Moses JW, Claessen B, Sanidas E, White HD, Ohman EM, et al. Impact of baseline thrombocytopenia on the early and late outcomes after ST-elevation myocardial infarction treated with primary angioplasty: analysis from the harmonizing outcomes with revascularization and stents in acute myocardial infarction (HORIZONS-AMI) trial. Am Heart J. 2011;161(2):391–6. doi:10.1016/j.ahj.2010.11.001.
- Park S, Ahn JM, Kim TO, Park H, Cho S-C, Kang D-Y, Lee PH, Park D-W, Park S-J. Incidence and impact of thrombocytopenia in patients undergoing percutaneous coronary intervention with drug-eluting stents. Am J Cardiol. 2020;134:55–61. doi:10.1016/j.amjcard.2020.07.059.
- Urban L, Ingrid Š, Žolková J, Ján S, Bolek T, Samoš M. High on-treatment platelet reactivity in patients undergoing complex percutaneous coronary interventions. Clin Appl Thromb Hemost. 2023;29:10760296231199089. doi:10.1177/10760296231199089.
- Siller-matula JM, Trenk D, Schrör K, Gawaz M, Kristensen SD, Storey RF, Huber K. Response variability to P2Y12 receptor inhibitors: expectations and reality. JACC Cardiovasc Interv. 2013;6(11):1111–28. doi:10.1016/j.jcin.2013.06.011.
- Siller-matula JM, Lang I, Christ G, Jilma B. Calcium-channel blockers reduce the antiplatelet effect of clopidogrel. J Am Coll Cardiol. 2008;52(19):1557–63. doi:10.1016/j.jacc.2008.07.055.