Abstract
Protein S (PS) is a vital endogenous anticoagulant. It plays a crucial role in regulating coagulation by acting as a cofactor for the activated protein C (APC) and tissue factor pathway inhibitor (TFPI) pathways. Additionally, it possesses direct anticoagulant properties by impeding the intrinsic tenase and prothrombinase complexes. Protein S oversees the coagulation process in both the initiation and propagation stages through these roles. The significance of protein S in regulating blood clotting can be inferred from the significant correlation between deficits in protein S and an elevated susceptibility to venous thrombosis. This is likely because activated protein C and tissue factor pathway inhibitor exhibit low efficacy as anticoagulants when no cofactors exist. The precise biochemical mechanisms underlying the roles of protein S cofactors have yet to be fully elucidated. Nevertheless, recent scientific breakthroughs have significantly enhanced comprehension findings for these functions. The diagnosis of protein S deficiency, both from a technical and genetic standpoint, is still a subject of debate due to the complex structural characteristics of the condition. This paper will provide an in-depth review of the molecular structure of protein S and its hemostatic effects. Furthermore, we shall address the insufficiency of protein S and its methods of diagnosis and treatment.
Plain Language Summary
What is the purpose of this summary?
To provide an in-depth review of the molecular structure of protein S and its hemostatic effects.
To address the deficiency of protein S and its methods of diagnosis and treatment.
What is known?
Protein S operates as an anticoagulant through its roles as a cofactor for APC, TFPI, and an inhibitor of FIXa.
Protein S deficiency can be either inherited or acquired.
What is new?
Plasma protein S and platelet-derived protein S contribute to regulating coagulation and maintaining hemostasis. Protein S can be used as a potential promising treatment target for persons diagnosed with hemophilia.
Introduction
Hemostasis is a complex and intricate physiological mechanism that limits bleeding from blood vessels in response to damage.Citation1 It involves the formation of blood clots to staunch bleeding and removing excess clots once the injury has healed.Citation2 The process of achieving sufficient hemostasis can be divided into multiple stages. It begins with forming the primary platelet plug, known as primary hemostasis.Citation3 This plug is further reinforced by synthesizing fibrin from the coagulation cascade, called secondary hemostasis.Citation4 Finally, any unnecessary thrombi are dissolved through fibrinolysis.Citation5 The process of primary hemostasis is initiated through the interaction between platelets and the vascular wall, facilitated by adhesive proteins, resulting in the formation of the initial platelet plug.Citation6 Most clotting factors in the bloodstream exist in an inactive state known as the zymogen form, and both intrinsic and extrinsic mechanisms facilitate their conversion into the active form.Citation7 The conversion of fibrinogen to insoluble fibrin and its subsequent stabilization by cross-linking by activated plasma FXIII required the presence of thrombin.Citation8 A precise homeostasis of several inhibitors regulates the coagulation process. The delicate balance is disrupted when there is an elevation in coagulation factors or a reduction in natural anticoagulant levels.Citation9
The primary mechanisms responsible for natural anticoagulation are antithrombin (AT), tissue factor pathway inhibitor (TFPI), and the protein C (PC) pathway.Citation10 AT is a highly effective inhibitor of serine proteases synthesized by the liver and distributed in plasma,Citation11 with a minimal quantity also present on the surface of endothelial cellsCitation12 and platelets.Citation13 This compound is a selective inhibitor of thrombin and factor Xa (FXa), effectively binding to coagulation factors, including VIIa, IXa, XIa, and XIIa, making them inactive.Citation14 Due to the low concentration of heparin in plasma, AT activation in the plasma is limited. However, binding AT with heparin sulfate on the surface of endothelial cells promotes their activation.Citation15 Following activation, the reticuloendothelial system eliminates the complex AT and its associated components, depleting the more rapidly forming complex.Citation16 Furthermore, TFPI is the second endogenous antithrombotic factor found on endothelial cells and platelets, associated with lipoproteins.Citation2 This compound strongly inhibits the FXa and tissue factor-factor VIIa (TF-FVIIa) complex.Citation17 The latest discovery reveals that protein S (PS) has been recognized as a critical cofactor for TFPI in inhibiting FXa, particularly in the presence of modest procoagulant stimuli. However, PS does not exhibit the same inhibitory effect on the TF-FVIIa complex.Citation18 PS is distributed throughout the plasma in conjunction with the TFPI, elevating their binding affinity for FXa and enhancing their stability within the plasma.Citation19
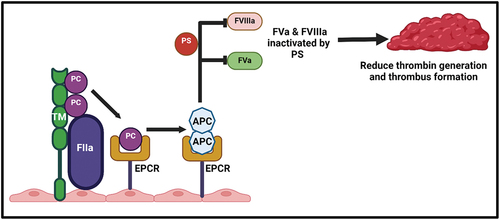
PC pathway is the third mechanism of spontaneous anticoagulation. The prothrombinase and tenase complexes are linked to factor VIIIa (FVIIIa) and factor Va (FVa) cofactors, respectively.Citation20 The activation of the PC in conjunction with PS has been observed to increase the inhibition of FVIIIa and FVa while simultaneously suppressing the actions of the tenase and prothrombinase complexes.Citation21 It is an anticoagulant factor and vitamin K-dependent protein composed of 419 amino acids biosynthesized by the liver.Citation22 Thrombin production via coagulation cascades steadily rises and subsequently interacts with the transmembrane protein thrombomodulin located on the endothelial surface.Citation23 Upon binding to thrombomodulin, activated protein C (APC) is generated and then attaches to the adjacent endothelial protein C receptor (EPCR), thereby facilitating the activation of more PC.Citation24 Following this, APC interacts with PS to create a complex known as APC-PS, which effectively inhibits the activity of FVIIIa and FVa ().Citation25
Figure 1. Protein C and protein S activation mechanisms. on the surface of endothelial cell membrane, thrombin (FIIa) binds to the thrombomodulin (TM) to form FIIa-TM complex, which activates protein C (APC). The affinity and efficiency of protein C is significantly increased after binding to endothelial protein C receptor (EPCR) and after dissociation their anticoagulant activity is increase. APC binds to protein S (PS) as a cofactor to form APC-PS complex and suppresses the tenase and prothrombinase activity by inhibiting FVa and FVIIIa and subsequently reducing the thrombin generation and thrombus formation.
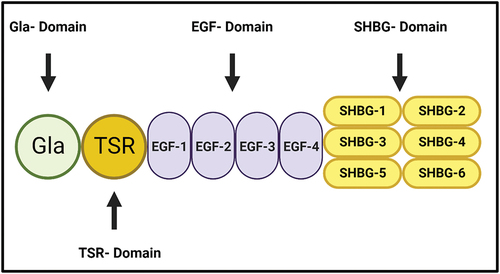
PS was discovered in 1977 in Seattle in the US, and it takes the letter (S) from this city as a new γ-carboxyglutamate (Gla) containing protein after purifying PC from bovine plasma.Citation26 It is a 73 kDa vitamin K-dependent glycoprotein circulating at 20–25 µg/ml concentration, mainly in the α platelet granules.Citation22 The molecular structure of PS consists of several domains, including the N-terminal Gla domain, a thrombin-sensitive region (TSR), four epidermal growth factor (EGF)-like domains and a sex hormone-binding globulin (SHBG)-like region, which is composed of two laminin G-type (LG) domains ().Citation27 These domains are crucial in PS interaction with other proteins and for other PS fundamental roles. For example, the Gla-domain is essential for the binding of PS to the negative charge phospholipid membrane, and the TSR domain stabilizes this binding and increases the stability of PS.Citation28 It is mainly synthesized and secreted by hepatocytes, endothelial cells and platelet α granules derived from the megakaryocytes.Citation29 It contains 676 amino acid precursor proteins and is circulated in the plasma in 60% bound to complement component 4 binding protein (C4BP), while the other 40% is circulated as free protein S (FPS).Citation30 The binding site of PS is located on the β-chain of C4BP, and the specific function of the PS- C4BP complex is still unknown.Citation31 This review will explain the molecular aspects of protein S and the main functions in the hemostasis mechanism. Also, we will describe the deficiency features with the diagnostic and clinical management of protein S deficiency patients.
Figure 2. The molecular Structure of Protein S (PS). PS consists of several domains, including the N-terminal Gla domain, a thrombin-sensitive region (TSR), four epidermal growth factor (EGF)-like domains and a sex hormone-binding globulin (SHBG)-like region, which is composed of two laminin G-type (LG) domains. It is essential for the interaction of protein S with the other proteins.
Protein S functions
PS is a multifunctional protein involved in various physiological processes such as hemostasis, inflammation, and other cellular mechanisms.Citation29 Within the context of hemostasis, PS anticoagulant plays a crucial role in the coagulation cascade.Citation23 It is a cofactor for APC,Citation32 TFPI and functions as a direct binding inhibitor for FIXa.Citation33
Protein S as a cofactor of activated protein C
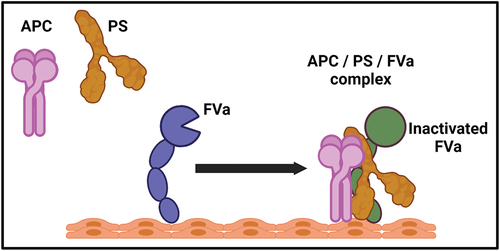
APC is a serine protease that inhibits the synthesis of thrombin during the propagation phase of the coagulation process. It deactivates the cofactors FVa and FVIIIa, which are involved in the activity of prothrombinase and tenase, respectively.Citation34 PS functions as a cofactor for the proteolytic processes and regulation of coagulation by binding to APC.Citation35 The initiation of this process occurs when thrombin activates FV to FVa and subsequently binds with FXa to create the prothrombinase complex.Citation36 The inhibitory effect of APC on FVa is mediated by its proteolytic activity.Citation37 At the same time, the presence of PS significantly enhances the rate of proteolysis by 20 to 30 folds.Citation38 Nevertheless, the molecular processes behind the ability of PS to improve this rate are currently being investigated.Citation39 Recently, a scientist has developed a concept regarding the potential enhancement of PS to the efficacy of APC. It has been proposed that the active site of APC will undergo a relocation close to the phospholipid membrane upon binding of PS to APC.Citation40 Also, the ability of FXa to protect FVa from the inhibitory action of APC is removed by PS.Citation37 Both ideas suggested that the presence of PS enhances the attraction of APC toward negatively charged surfaces due to phospholipids.Citation39 The research conducted by Smirnov et al. presented a set of hypotheses, demonstrating that the coexistence of PS and FVa led to a significant enhancement in APC efficiency (). Furthermore, the recruitment of APC to the phospholipid surface, which is necessary for the interaction with FVa, was significantly enhanced by more than 14 times in the presence of both PS and FVa.Citation41 The findings presented in this study provide compelling evidence for the existence of the APC/PS/FVa complex and its role in inhibiting and regulating the coagulation process.Citation42 Regarding FVIIIa, it is essential to note that PS is a cofactor for APC in the process of inactivating FVIIIa.Citation32 This interaction leads to a reduction in thrombin generation and the formation of fibrin.Citation43 Additionally, it is worth mentioning that in the presence of PS and APC, there is a synergistic effect from FVa for the inactivation of FVIIIa.Citation44 The precise molecular mechanism behind the participation of PS in the inactivation of FVIIIa remains unclear.Citation43
Figure 3. The enhancement of activated protein C with cofactor protein S and FVa. the efficiency of APC is significantly increase in the presence of protein S and FVa together. The recruitment of APC to the phospholipid surface required for FVa and the efficiency of APC increased in the presence of both PS and FVa. The formation of APC/PS/FVa complex is crucial for the regulation of coagulation mechanisms.
Protein S as a cofactor of tissue factor Pathway Inhibitor and direct inhibitor of FIXa activity
TFPI is a glycoprotein that consists of a single chain and contains a proteinase inhibitor domain.Citation45,Citation46 It is present in plasma at relatively low levels.Citation46 There are two isoforms, TFPIα, held in platelets and released when activated,Citation19 and TFPIβ, linked to endothelial cells.Citation47 This compound directly inhibits the activity of the free FXa and the TF-FVIIa complex.Citation48 PS play a crucial role in modulating the coagulation cascade via the extrinsic pathway, primarily by acting as a cofactor for TFPI.Citation49 PS is a cofactor for the TFPIα isoform, which can be found in plasma or secreted from platelets. However, it does not influence the TFPIβ isoform.Citation50 The concentration of the TFPIα isoform in plasma is correlated with the concentration of PS.Citation18 This correlation has been seen in patients with a quantitative deficiency in PS, who also exhibit a decrease in the concentration of TFPIα isoform. This connection implies a strong interaction between PS and the TFPIα isoform in plasma.Citation51 In basic terms, the association between PS and TFPIα isoform involves the suppression of FXa. Nonetheless, the complete molecular mechanisms behind this functionality remain undiscovered.Citation52
Factor IX (FIX) is a zymogen, which is a 55-kDa single-chain polypeptide and present in the plasma at a concentration of 70–90 nM.Citation53 When FVIIIa is present, FIX is activated to FIXa and triggers the activation of FX to FXa which is crucial in the intrinsic coagulation pathway.Citation54 Recently, PS has been found to have an additional role by directly binding and inhibiting of FIXa, whether FVIIIa is present or not.Citation55 This novel function of PS is essential for the maximal regulation of thrombin generation in the presence of FIXa and FVIIIa.Citation56 The involvement of FIXa in this context was identified through in vivo experiments that demonstrated its direct interaction with the K132, K126, and R170 amino acid residues in the FIXa heparin-binding exosite. This interaction suppressed the intrinsic Xase complex and subsequent regulation of thrombin production.Citation57 Interestingly, Hemophilia B mice that were given an infusion of a FIXa double mutant, specifically K132A/R170A, which had complete functionality but was unable to bind to PS, showed a faster rate of fibrin clot formation compared to animals that were infused with the wild type FIXa. Significantly, the interruption of PS and FIXa binding resulted in an elevated rate of blood clot formation in mice with Hemophilia B.Citation57 These findings demonstrated that PS-FIXa interaction is a significant regulating mechanism for thrombin generation and thrombus formation.Citation58
Gene regulation of PS and their hemostatic regulation
PS is observed in genomic DNA, and it’s located on chromosome 3. This chromosome is comprised of a total of 15 exons and 14 introns.Citation59 The gene encoding PS contains specific binding sites for transcription factors, including Sp1.Citation60 It has been hypothesized that deregulating these binding sites may lead to aberrant upregulation of PS in individuals affected by cancer. Furthermore, the expression of PS can be regulated by many hormones, including progesterone and estrogen.Citation61 The progesterone can promote the expression of PS by approximately 25%.Citation62 However, it is downregulated by estrogen because the ER binding to promoter-distal GC-rich motifs of the PS gene is promoting.Citation29 Resulted of that RIP140 and HDA3 complexes that deacetylate histones can recruited which increase the risk of developing deep vein thrombosis (DVT) in the pregnant women.Citation63 Interleukin-6 (IL-6) is an additional regulator of PS expression throughout the inflammatory process, playing a crucial role in regulating inflammation.Citation64 Nevertheless, any genetic mutation can influence the expression and function of protein S.Citation65
As previously indicated, PS play a pivotal function in regulating the coagulation process. The presence of PS deficiency has contributed to an increased risk of developing venous thromboembolism (VTE), particularly DVT.Citation66 A recent study demonstrated that platelet-derived protein S, can modulate thrombus formation in condition of low-shear stress, with the involvement of APC and TFPI as cofactors. The results of this study indicated that both plasma PS and platelet-derived PS may play a role in regulating the coagulation process and maintaining hemostasis. However, the specific method by which platelet-derived PS does this function remains unclear.Citation67 Conversely, PS exhibits promise as a prospective therapeutic target for individuals diagnosed with hemophilia.Citation68 Currently, multiple target medicines for TFPI are being evaluated in clinical trials.Citation69 However, PS exhibits more value than TFPI therapies due to its multifunctional involvement in coagulation.Citation68
Protein S deficiency
PS deficiency may occur as either an inherited condition or acquired due to external factors. Hereditary PS deficiency is an uncommon yet significant autosomal dominant condition exhibiting symptoms similar to other coagulation inhibitors.Citation70 The predominant clinical manifestations in individuals with heterozygous PS deficiency are DVT and pulmonary embolism (PE).Citation71 Instances of homozygous and compound heterozygous PS deficiencies are infrequently documented,Citation72 and in most cases, they are linked to the development of severe purpura fulminans during the newborn stage.Citation73 The exact incidence of PS deficiency in the general population is uncertain; however, it is acknowledged to be minimal.Citation74
Approximately 300 mutations in PS have been found and categorized, resulting in the classification of PS deficiency into three distinct types.Citation75 The Type I deficiency results from modifying the Sp1 transcription factor binding within the promoter region, reducing the overall PS levels.Citation76 In cases of type II deficiency, mutations in the propeptide (specifically Arg40Leu and Arg40Leu) prohibit the PS from maturing. It leads to changes in the cofactor APC. However, the overall quantity of PS remains unaffected.Citation77 Furthermore, in cases of type III deficit, the overall amount of PS remains the same. However, FPS levels and the activity of APC are diminished.Citation78 One of the highly significant mutation in PS, known as PS-Tokushima, is associated with a genetic risk factor for DVT in the Japanese population.Citation79 In this mutation, the second PS epidermal growth factor-like (EGF) domain contained the variant p.Lys196Glu, K196E, which was overlooked.Citation80 The frequency of this particular variant was determined to be approximately 1.65–1.8% among the whole Japanese population.Citation81 However, the presence of this condition has not yet been detected in the Chinese, Korean, or Caucasian populations.Citation82
PS deficiency can be acquired due to factors such as kidney illness, cancer, pregnancy, or the use of oral contraceptives containing estrogen.Citation83 Data indicates that PS deficiency contributes to increased blood clotting in these individuals.Citation84 Acquired PS deficiency is observed in women with elevated estrogen levels, and the underlying process is currently unknown.Citation85 The risk of loss recurrent pregnancy is 15-fold increases in women with PS deficiency which recommend the thrombophilia screening tests and treatment with low molecular weight heparin (LMWH).Citation86 In normal pregnancy, the coagulation profiles and thrombophilia markers during the three trimesters should be monitoring because it could be changed and may help doctor effectively to diagnose and treat any conditions may arise during pregnancy.Citation87 Furthermore, sickle cell anemia is another factor that can lead to acquired PS deficiency, increasing the patient’s susceptibility to thromboembolic diseases.Citation88 Moreover, hypoxia can reduce the production of PS by stabilizing the typical hypoxia response in the liver known as HIF1α, which in turn promotes the development of thrombosis.Citation89 Additionally, human immunodeficiency virus (HIV) infection or multiple myeloma (MM) can also cause acquired autoimmune PS deficiency.Citation90 The other pathological factors, such as disseminated intravascular coagulopathy (DIC) and oral anticoagulant medication, such as warfarin, are associated with acquired PS deficiency.Citation91 The association between warfarin and acquired PS deficiency should be considered during the treatment regime of thrombosis in PS-deficient patients.Citation92 Basically, warfarin is the predominant anticoagulant used for the treatment of DVT or PE.Citation93 The injectable anticoagulants such as heparin and LMWH must be administered with warfarin until it reaches its full effectiveness.Citation94 In patients with PS deficiency, warfarin must be use with other anticoagulant such as LMWH because warfarin alone can inhibit the production of PS and may exacerbate the current blood clots or trigger a new clots or a severe skin rash called skin necrosis.Citation92
Diagnosis and clinical management of protein S deficiency
The technical and genetic diagnosis of PS deficiency remains debatable due to its presence in two forms; free protein S (FPS) and a complex with C4BP, forming the PS-C4BPβ+ complex. The structural composition of PS adds complexity to antigenic and functional determination.Citation95 Moreover, there exists a significant convergence between the levels of PS in normal and heterozygous persons, and these levels tend to vary periodically. Furthermore, conventional methods for measuring unbound PS tend to have a high degree of inaccuracy.Citation96 A new assay that utilizes the strong binding between PS and C4BP has recently been demonstrated to be a highly accurate and trustworthy test.Citation97 The research indicates that there are inaccurately high measurements of FPS when the test temperatures are increased. This could be caused by a temperature-related rise in separating the PS-C4BPβ+ complex in samples lacking PS.Citation98
Plasma PS levels vary depending on age, gender, and inherited or acquired variables, such as hormone state or lipid metabolism.Citation99 PS levels, both total and free, are comparatively lower in women than in males. However, as individuals age, total PS levels tend to increase. This age-related difference is more noticeable in women due to variations in hormone levels. Age does not impact the levels of PS that are available without restriction. Significantly, patients with factor V Leiden, which disrupts PC function, may exhibit an erroneously low level of functional PS.Citation100 Several novel commercial techniques are now accessible for accurately identifying PS deficiency in factor V Leiden through the dilution of test plasma.Citation101 The performance of total PS test is outstanding. However, they cannot identify PS deficit of types 2 and 3. FPS tests can be a valuable alternative despite their limited consistency. APC cofactor activity measurement could serve as an alternative biomarker of PS insufficiency, notwithstanding the high likelihood of false-positive results in these assays.Citation102 Examining mutations in the PROS1 gene is crucial for detecting PS deficiency, which can be achieved using DNA sequencing or amplification and subsequent analysis using polymerase chain reaction (PCR) followed by gel electrophoresis.Citation103 Possible alternative diagnoses that can be considered in people with PS deficit involve antiphospholipid syndrome, AT deficiency, factor 5 Leiden mutation, PC deficiency, malignancy, prothrombin gene mutation, and paroxysmal nocturnal hemoglobinuria (PNH).Citation104
PS deficiency presents as thrombophilia, characterized by an imbalance of coagulation components, resulting in irregular blood clotting. This pathophysiological disruption places patients vulnerable to developing DIC, which can lead to severe conditions such as cerebrovascular damage or persistent VTE.Citation105 The management of VTE involves the use of anticoagulation medications, such as heparin either LMWH or unfractionated heparin, vitamin K antagonist (VKA) or direct oral anticoagulants (DOACs).Citation94 DOACs are anticoagulants used to prevent blood clot formation and it is alternative to the oral VKA (warfarin). It is classified into two primary categories depending on its method of action, which involves directly inhibiting coagulation factors. Oral direct factor Xa inhibitors include rivaroxaban, apixaban, edoxaban, and betrixaban. The second type acts as a direct inhibitor of thrombin (FIIa), such as dabigatran. The selection between the DOACs and VKA is based on the patient’s personal preference and level of efficiency. Previously, VKA was the preferred medication for treating VTE, but this has shifted with the introduction of DOACs.Citation106 Due to their proven effectiveness and favorable safety profile, DOACs are used more frequently to treat VTE. In cohort research involving individuals with hereditary thrombophilia, DOACs have demonstrated comparable efficacy to heparin/VKA. However, DOACs were associated with a higher likelihood of non-major bleeding, whereas VKA exhibited a slightly elevated risk of substantial bleeding.Citation94 Individuals afflicted with congenital PS deficiency often have an extended course of anticoagulation treatment until their coagulation activity is stable for at least two consecutive days. If the initial thrombotic episode is life-threatening or occurs in several atypical locations, such as brain veins, it is advisable to undergo lifelong therapy. If the thrombotic episode is caused by a significant trauma or surgery and the thrombosis is not life-threatening, it is not advisable.Citation93 Patients with PS deficiency who are exposed to thrombotic risk factors, such as air travel, surgery, pregnancy, or prolonged immobilization, should get prophylactic medication. Pregnant individuals in the initial trimester or after 36 weeks should get LMWH instead of warfarin to minimize the chances of bleeding in both the fetus and the mother.Citation107
Conclusion and future perspectives
The presence of a hypercoagulable state in individuals with a congenital deficit of PS highlights the critical role of this protein in regulating blood clotting. PS operates as an anticoagulant through its roles as a cofactor for APC, TFPI, and an inhibitor of FIXa. Hepatocytes, endothelial cells, and platelet α granules produced from megakaryocytes are the primary sources of synthesis and secretion of this substance. Progesterone can increase PS expression, whereas estrogen, pregnancy-related thrombosis, and interleukin-6 during inflammation can downregulate it. However, any genetic mutation can affect the expression and function of PS. Both plasma PS and platelet-derived PS likely contribute to regulating coagulation and maintaining hemostasis. The exhibits show potential as a promising treatment target for persons diagnosed with hemophilia. In addition, PS deficiency can be either inherited or acquired. Deep vein thrombosis and pulmonary embolism are the main clinical symptoms observed in persons with heterozygous PS deficiency. PS deficiency may be acquired due to conditions such as cancer and pregnancy. The diagnosis of PS deficiency is challenging due to the complexity in their structure. PS deficiency can be managed using the same approach as VTE, which entails the administration of anticoagulant medications.
The significant role of PS as an anticoagulant protein has been well-established in clinical practice.Citation22 We understand that it plays a crucial role as a cofactor in two of our anticoagulant cascades by improving the activities of APC and TFPIα.Citation108 A distinct pattern is becoming evident, where PS and different forms of FV contribute to the development of beneficial interactions in the anticoagulant pathway.Citation42 Based on the prior findings, further research is necessary to explore recent advancements in PS clinical research related to pregnancy and managing PS shortage. Furthermore, additional study is required to explore the potential association between COVID-19 morbidity and PS insufficiency. The precise functions of plasma and platelet PS in regulating thrombin production within and outside the platelet thrombus are yet to be established. Additional, comprehensive molecular explanations of the numerous connections associated with the diverse functions of PS are required. Additionally, they could serve as a foundation for comprehending how dynamic and flow variables influence the functional and clinical efficacy of PS as a regulator of hemostasis.
Authors contributions
S.A. Designed the review, wrote the manuscript and drew the figures, A.A.B and I.A.A. collected and drafted the relevant literature review part, and S.K.A. revised the manuscript.
All authors have read and agreed to the published version of the manuscript.
Acknowledgments
We acknowledge King Faisal Medical City for Southern Region (KFMCity), King Fahad Medical City (KFMC) and King Saud University (KSU) for their support.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Schiffman FJ. Hematologic pathophysiology. In: The Clinical Pathophysiology. 5th ed. 1998. p. 75–102.
- Broze GJ Jr. Tissue factor pathway inhibitor and the revised theory of coagulation. Annu Rev Med. 1995;46(1):103–8. doi:10.1146/annurev.med.46.1.103.
- Livio M, Vigano G, Morigi M, Ubiali A, Galbusera M, Remuzzi G. Role of platelet-activating factor in primary hemostasis. Am J Physiol Heart Circ Physiol. 1988;254(6):H1218–23. doi:10.1152/ajpheart.1988.254.6.H1218.
- Lawkowicz W, Michalowska R, Slomkowski M. Disturbances in hemostasis in secondary thrombocytopenia in the light of studies on platelet aggregation. Pol Arch Med Wewn. 1969;43:1555–62.
- Faulk WP, Labarrere CA, Nelson DR, Pitts D. Hemostasis, fibrinolysis, and natural anticoagulation in transplant vascular sclerosis. J Heart Lung Transplant. 1995;14:S158–64.
- Yamazaki H, Fujimoto T, Suzuki H, Akamatsu N, Katagiri Y, Yamaguchi A, Tanoue K. Interaction of platelets and blood vessels–vascular injuries induced by platelet activation in vivo. Jpn Circ J. 1992;56(2):178–86. doi:10.1253/jcj.56.178.
- Cugno M, Cicardi M, Bottasso B, Coppola R, Paonessa R, Mannucci PM, Agostoni A. Activation of the coagulation cascade in C1-inhibitor deficiencies. Blood. 1997;89(9):3213–8. doi:10.1182/blood.V89.9.3213.
- Mertens K, Bertina RM. Activation of human coagulation factor VIII by activated factor X, the common product of the intrinsic and the extrinsic pathway of blood coagulation. Thromb Haemost. 1982;47(2):96–100. doi:10.1055/s-0038-1657137.
- Tomaiuolo M, Brass LF, Stalker TJ. Regulation of platelet activation and coagulation and its role in vascular injury and arterial thrombosis. Interv Cardiol Clin. 2017;6(1):1–12. doi:10.1016/j.iccl.2016.08.001.
- Colvin BT. Physiology of haemostasis. Vox Sang. 2004;87(Suppl 1):43–6. doi:10.1111/j.1741-6892.2004.00428.x.
- Roemisch J, Gray E, Hoffmann JN, Wiedermann CJ. Antithrombin: a new look at the actions of a serine protease inhibitor. Blood Coagul Fibrinolysis. 2002;13(8):657–70. doi:10.1097/00001721-200212000-00001.
- Justus AC, Roussev R, Norcross JL, Faulk WP. Antithrombin binding by human umbilical vein endothelial cells: effects of exogenous heparin. Thromb Res. 1995;79(2):175–86. doi:10.1016/0049-3848(95)00103-X.
- Ishida Y, Yano K, Ito T, Shigematus H, Sasaki K, Kondo S, Kuriya S-I. Purification of proplatelet formation (PPF) stimulating factor: thrombin/antithrombin III complex stimulates PPF of megakaryocytes in vitro and platelet production in vivo. Thromb Haemost. 2001;85(2):349–55. doi:10.1055/s-0037-1615691.
- He S, Bremme K, Blomback M. Acquired deficiency of antithrombin in association with a hypercoagulable state and impaired function of liver and/or kidney in preeclampsia. Blood Coagul Fibrinolysis. 1997;8(4):232–8. doi:10.1097/00001721-199706000-00004.
- Opal SM, Kessler CM, Roemisch J, Knaub S. Antithrombin, heparin, and heparan sulfate. Crit Care Med. 2002;30(5 Suppl):S325–31. doi:10.1097/00003246-200205001-00024.
- Hermann H, Galloni L, Cier JF. Is antithrombin formed by the reticuloendothelial tissue? C R Seances Soc Biol Fil. 1945;139:1134.
- Osterud B, Bajaj MS, Bajaj SP. Sites of tissue factor pathway inhibitor (TFPI) and tissue factor expression under physiologic and pathologic conditions. On behalf of the subcommittee on tissue factor pathway inhibitor (TFPI) of the scientific and standardization committee of the ISTH. Thromb Haemost. 1995;73(5):873–5. doi:10.1055/s-0038-1653884.
- Ellery PER, Hilden I, Sejling K, Loftager M, Martinez ND, Maroney SA, Mast AE. Correlates of plasma and platelet tissue factor pathway inhibitor, factor V, and protein S. Res Pract Thromb Haemost. 2018;2(1):93–104. doi:10.1002/rth2.12058.
- Kato H. Tissue factor pathway inhibitor; its structure, function and clinical significance. Pol J Pharmacol. 1996;48:67–72.
- van ‘t Veer C, Butenas S, Golden N, Mann K. Regulation of prothrombinase activity by protein S. Thromb Haemost. 1999;82(1):80–7. doi:10.1055/s-0037-1614633.
- Kim PY, Nesheim ME. Down regulation of prothrombinase by activated protein C during prothrombin activation. Thromb Haemost. 2010;104(1):61–70. doi:10.1160/TH09-09-0650.
- Dahlback B. Vitamin K-dependent protein S: beyond the protein C pathway. Semin Thromb Hemost. 2018;44(2):176–84. doi:10.1055/s-0037-1604092.
- Walker FJ. Regulation of activated protein C by a new protein. A possible function for bovine protein S. J Biol Chem. 1980;255(12):5521–4. doi:10.1016/S0021-9258(19)70660-7.
- Xu J, Esmon NL, Esmon CT. Reconstitution of the human endothelial cell protein C receptor with thrombomodulin in phosphatidylcholine vesicles enhances protein C activation. J Biol Chem. 1999;274(10):6704–10. doi:10.1074/jbc.274.10.6704.
- Esmon CT. Protein C anticoagulant pathway and its role in controlling microvascular thrombosis and inflammation. Crit Care Med. 2001;29(7 Suppl):S48–51; discussion 51–2. doi:10.1097/00003246-200107001-00018.
- Di Scipio RG, Hermodson MA, Yates SG, Davie EW. A comparison of human prothrombin, factor IX (Christmas factor), factor X (Stuart factor), and protein S. Biochemistry. 1977;16(4):698–706. doi:10.1021/bi00623a022.
- Saller F, Villoutreix BO, Amelot A, Kaabache T, Le Bonniec BF, Aiach M, Gandrille S, Borgel D. The γ-carboxyglutamic acid domain of anticoagulant protein S is involved in activated protein C cofactor activity, independently of phospholipid binding. Blood. 2005;105(1):122–30. doi:10.1182/blood-2004-06-2176.
- Borgel D, Gaussem P, Garbay C, Bachelot-Loza C, Kaabache T, Liu W-Q, Brohard-Bohn B, Le Bonniec B, Aiach M, Gandrille S. et al. Implication of protein S thrombin-sensitive region with membrane binding via conformational changes in the γ-carboxyglutamic acid-rich domain. Biochem J. 2001;360(2):499–506. doi:10.1042/bj3600499.
- Suleiman L, Negrier C, Boukerche H. Protein S: a multifunctional anticoagulant vitamin K-dependent protein at the crossroads of coagulation, inflammation, angiogenesis, and cancer. Crit Rev Oncol Hematol. 2013;88(3):637–54. doi:10.1016/j.critrevonc.2013.07.004.
- Dahlback B. Inhibition of protein Ca cofactor function of human and bovine protein S by C4b-binding protein. J Biol Chem. 1986;261(26):12022–7. doi:10.1016/S0021-9258(18)67196-0.
- Schwalbe R, Dahlbäck B, Hillarp A, Nelsestuen G. Assembly of protein S and C4b-binding protein on membranes. J Biol Chem. 1990;265(27):16074–81. doi:10.1016/S0021-9258(17)46190-4.
- Walker FJ, Chavin SI, Fay PJ. Inactivation of factor VIII by activated protein C and protein S. Arch Biochem Biophys. 1987;252(1):322–8. doi:10.1016/0003-9861(87)90037-3.
- Heeb MJ. et al. Protein S binds to and inhibits factor Xa. Proc Natl Acad Sci USA. 1994;91(7):2728–32. doi:10.1073/pnas.91.7.2728.
- Dahlback B. Pro- and anticoagulant properties of factor V in pathogenesis of thrombosis and bleeding disorders. Int J Lab Hematol. 2016;38(Suppl 1):4–11. doi:10.1111/ijlh.12508.
- Walker FJ. Regulation of activated protein C by protein S. The role of phospholipid in factor Va inactivation. J Biol Chem. 1981;256(21):11128–31. doi:10.1016/S0021-9258(19)68566-2.
- Bos MH, Camire RM. A bipartite autoinhibitory region within the B-domain suppresses function in factor V. J Biol Chem. 2012;287(31):26342–51. doi:10.1074/jbc.M112.377168.
- Norstrom EA, Tran S, Steen M, Dahlbäck B. Effects of factor Xa and protein S on the individual activated protein C-mediated cleavages of coagulation factor Va. J Biol Chem. 2006;281(42):31486–94. doi:10.1074/jbc.M606441200.
- Maurissen LF, Thomassen MCLGD, Nicolaes GAF, Dahlbäck B, Tans G, Rosing J, Hackeng TM. Re-evaluation of the role of the protein S-C4b binding protein complex in activated protein C-catalyzed factor Va-inactivation. Blood. 2008;111(6):3034–41. doi:10.1182/blood-2007-06-089987.
- Gierula M, Ahnstrom J. Anticoagulant protein S-New insights on interactions and functions. J Thromb Haemost. 2020;18(11):2801–11. doi:10.1111/jth.15025.
- Yegneswaran S, Smirnov MD, Safa O, Esmon NL, Esmon CT, Johnson AE. Relocating the active site of activated protein C eliminates the need for its protein S cofactor. A fluorescence resonance energy transfer study. J Biol Chem. 1999;274(9):5462–8. doi:10.1074/jbc.274.9.5462.
- Smirnov MD, Safa O, Regan L, Mather T, Stearns-Kurosawa DJ, Kurosawa S, Rezaie AR, Esmon NL, Esmon CT. A chimeric protein C containing the prothrombin Gla domain exhibits increased anticoagulant activity and altered phospholipid specificity. J Biol Chem. 1998;273(15):9031–40. doi:10.1074/jbc.273.15.9031.
- Gierula M, Salles‐Crawley II, Santamaria S, Teraz‐Orosz A, Crawley JTB, Lane DA, Ahnström J. The roles of factor Va and protein S in formation of the activated protein C/protein S/factor Va inactivation complex. J Thromb Haemost. 2019;17(12):2056–68. doi:10.1111/jth.14594.
- Gale AJ, Cramer TJ, Rozenshteyn D, Cruz JR. Detailed mechanisms of the inactivation of factor VIIIa by activated protein C in the presence of its cofactors, protein S and factor V. J Biol Chem. 2008;283(24):16355–62. doi:10.1074/jbc.M708985200.
- Nyberg P, Dahlback B, Garcia de Frutos P. The SHBG-like region of protein S is crucial for factor V-dependent APC-cofactor function. FEBS Lett. 1998;433(1–2):28–32. doi:10.1016/S0014-5793(98)00877-1.
- Broze GJ Jr., Girard TJ. Tissue factor pathway inhibitor: structure-function. Front Biosci. 2012;17(1):262–80. doi:10.2741/3926.
- Bajaj MS, Birktoft J, Steer S, Bajaj SP. Structure and biology of tissue factor pathway inhibitor. Thromb Haemost. 2001;86(4):959–72. doi:10.1055/s-0037-1616518.
- Lwaleed BA, Bass PS. Tissue factor pathway inhibitor: structure, biology and involvement in disease. J Pathol. 2006;208(3):327–39. doi:10.1002/path.1871.
- Mast AE. Tissue factor pathway inhibitor: multiple anticoagulant activities for a single protein. Arterioscler Thromb Vasc Biol. 2016;36(1):9–14. doi:10.1161/ATVBAHA.115.305996.
- Tchaikovski SN, Thomassen MC, Costa S-D, Peeters L, Rosing J. Role of protein S and tissue factor pathway inhibitor in the development of activated protein C resistance early in pregnancy in women with a history of preeclampsia. Thromb Haemost. 2011;106(11):914–21. doi:10.1160/TH11-04-0244.
- Wood JP, Ellery PER, Maroney SA, Mast AE. Protein S is a cofactor for platelet and endothelial tissue factor pathway inhibitor-α but not for cell surface–associated tissue factor pathway inhibitor. Arterioscler Thromb Vasc Biol. 2014;34(1):169–76. doi:10.1161/ATVBAHA.113.302655.
- Castoldi E, Simioni P, Tormen ED, Rosin GJ, Hackeng TM. Hereditary and acquired protein S deficiencies are associated with low TFPI levels in plasma. J Thromb Haemost. 2010;8(2):294–300. doi:10.1111/j.1538-7836.2009.03712.x.
- Reglinska-Matveyev N, Andersson HM, Rezende SM, Dahlbäck B, Crawley JTB, Lane DA, Ahnström J. TFPI cofactor function of protein S: essential role of the protein S SHBG-like domain. Blood. 2014;123(25):3979–87. doi:10.1182/blood-2014-01-551812.
- Taran LD. Factor IX of the blood coagulation system: a review. Biochemistry (Mosc). 1997;62:685–93.
- Palta S, Saroa R, Palta A. Overview of the coagulation system. Indian J Anaesth. 2014;58(5):515–23. doi:10.4103/0019-5049.144643.
- Chattopadhyay R, Sengupta T, Majumder R. Inhibition of intrinsic Xase by protein S: a novel regulatory role of protein S independent of activated protein C. Arterioscler Thromb Vasc Biol. 2012;32(10):2387–93. doi:10.1161/ATVBAHA.112.250928.
- Plautz WE, Chattopadhyay R, Goldfeld EI, Samelson-Jones BJ, Pilli VS, Campello E, Datta A, Arruda VR, Simioni P, Majumder R. Padua FIXa resistance to protein S and a potential therapy for hyperactive FIXa. Thromb Res. 2018;170:133–41. doi:10.1016/j.thromres.2018.08.018.
- Plautz WE, Sekhar Pilli VS, Cooley BC, Chattopadhyay R, Westmark PR, Getz T, Paul D, Bergmeier W, Sheehan JP, Majumder R. Anticoagulant protein S targets the factor IXa heparin-binding exosite to prevent thrombosis. Arterioscler Thromb Vasc Biol. 2018;38(4):816–28. doi:10.1161/ATVBAHA.117.310588.
- Pilli VS, Plautz W, Majumder R. The journey of protein S from an anticoagulant to a signaling molecule. JSM Biochem Mol Biol. 2016;3(1):187–31.
- Schmidel DK, Tatro AV, Phelps LG, Tomczak JA, Long GL. Organization of the human protein S genes. Biochemistry. 1990;29(34):7845–52. doi:10.1021/bi00486a010.
- Watkins PC, Eddy R, Fukushima Y, Byers MG, Cohen EH, Dackowski WR, Wydro RM, Shows TB. The gene for protein S maps near the centromere of human chromosome 3. Blood. 1988;71(1):238–41. doi:10.1182/blood.V71.1.238.238.
- Sankpal UT, Goodison S, Abdelrahim M, Basha R. Targeting Sp1 transcription factors in prostate cancer therapy. Med Chem. 2011;7(5):518–25. doi:10.2174/157340611796799203.
- Hughes Q, Watson M, Cole V, Sayer M, Baker R, Staton J. Upregulation of protein S by progestins. J Thromb Haemost. 2007;5(11):2243–9. doi:10.1111/j.1538-7836.2007.02730.x.
- Dahlback B, Villoutreix BO. Regulation of blood coagulation by the protein C anticoagulant pathway: novel insights into structure-function relationships and molecular recognition. Arterioscler Thromb Vasc Biol. 2005;25(7):1311–20. doi:10.1161/01.ATV.0000168421.13467.82.
- Castoldi E, Hackeng TM. Regulation of coagulation by protein S. Curr Opin Hematol. 2008;15(5):529–36. doi:10.1097/MOH.0b013e328309ec97.
- Zhang K, Kurachi S, Kurachi K. Genetic mechanisms of age regulation of protein C and blood coagulation. J Biol Chem. 2002;277(6):4532–40. doi:10.1074/jbc.M109524200.
- Tassin M, Llarena P, Laffaye F, Kaltenbach G. Protein C deficiency in patient with sepsis-associated disseminated intravascular coagulation and deep vein thrombosis. Arch Argent Pediatr. 2013;111(1):e28–30. doi:10.5546/aap.2013.e28.
- Calzavarini S, Prince-Eladnani R, Saller F, Bologna L, Burnier L, Brisset AC, Quarroz C, Reina Caro MD, Ermolayev V, Matsumura Y, et al. Platelet protein S limits venous but not arterial thrombosis propensity by controlling coagulation in the thrombus. Blood. 2020;135(22):1969–82. doi:10.1182/blood.2019003630.
- Prince R, Bologna L, Manetti M, Melchiorre D, Rosa I, Dewarrat N, Suardi S, Amini P, Fernández JA, Burnier L, et al. Targeting anticoagulant protein S to improve hemostasis in hemophilia. Blood. 2018;131(12):1360–71. doi:10.1182/blood-2017-09-800326.
- Chowdary P. Anti-tissue factor pathway inhibitor (TFPI) therapy: a novel approach to the treatment of haemophilia. Int J Hematol. 2020;111(1):42–50. doi:10.1007/s12185-018-2548-6.
- Lane DA, Mannucci PM, Bauer KA, Bertina RM, Bochkov NP, Boulyjnkov V, Chandy M, Dahlback B, Ginter EK, Miletich JP, et al. Inherited thrombophilia: part 1. Thromb Haemost. 1996;76(5):651–62. doi:10.1055/s-0038-1650638.
- Kearon C, Crowther M, Hirsh J. Management of patients with hereditary hypercoagulable disorders. Annu Rev Med. 2000;51(1):169–85. doi:10.1146/annurev.med.51.1.169.
- Pung-Amritt P, Poort S, Vos H, Bertina R, Mahasandana C, Tanphaichitr V, Veerakul G, Kankirawatana S, Suvatte V. Compound heterozygosity for one novel and one recurrent mutation in a Thai patient with severe protein S deficiency. Thromb Haemost. 1999;81(2):189–92. doi:10.1055/s-0037-1614440.
- Mahasandana C, Suvatte V, Marlar R, Manco-Johnson M, Jacobson L, Hathaway W. Neonatal purpura fulminans associated with homozygous protein S deficiency. Lancet. 1990;335(8680):61–2. doi:10.1016/0140-6736(90)90201-F.
- Rosendaal FR. Venous thrombosis: a multicausal disease. Lancet. 1999;353(9159):1167–73. doi:10.1016/S0140-6736(98)10266-0.
- Gandrille S, Borgel D, Sala N, Espinosa-Parrilla Y, Simmonds R, Rezende S, Lind B, Mannhalter C, Pabinger I, Reitsma PH, et al. Protein S deficiency: a database of mutations–summary of the first update. Thromb Haemost. 2000;84(5):918. doi:10.1055/s-0037-1614137.
- Espinosa-Parrilla Y, Morell M, Borrell M, Souto JC, Fontcuberta J, Estivill X, Sala N. Optimization of a simple and rapid single-strand conformation analysis for detection of mutations in the PROS1 gene: identification of seven novel mutations and three novel, apparently neutral, variants. Hum Mutat. 2000;15(5):463–73. doi:10.1002/(SICI)1098-1004(200005)15:5<463:AID-HUMU8>3.0.CO;2-E.
- Gandrille S, Aiach M. Identification of mutations in 90 of 121 consecutive symptomatic French patients with a type I protein C deficiency. The French INSERM network on molecular abnormalities responsible for protein C and protein S deficiencies. Blood. 1995;86(7):2598–605. doi:10.1182/blood.V86.7.2598.2598.
- Garcia de Frutos P, Fuentes-Prior P, Hurtado B, Sala N. Molecular basis of protein S deficiency. Thromb Haemost. 2007;98(3):543–56. doi:10.1160/TH07-03-0199.
- Hayashi T, Nishioka J, Shigekiyo T, Saito S, Suzuki K. Protein S Tokushima: abnormal molecule with a substitution of Glu for Lys-155 in the second epidermal growth factor-like domain of protein S. Blood. 1994;83(3):683–90. doi:10.1182/blood.V83.3.683.683.
- Yamazaki T, Sugiura I, Matsushita T, Kojima T, Kagami K, Takamatsu J, Saito H. A phenotypically neutral dimorphism of protein S: the substitution of Lys155 by Glu in the second EGF domain predicted by an a to G base exchange in the gene. Thromb Res. 1993;70(5):395–403. doi:10.1016/0049-3848(93)90081-X.
- Kimura R, Honda S, Kawasaki T, Tsuji H, Madoiwa S, Sakata Y, Kojima T, Murata M, Nishigami K, Chiku M, et al. Protein S–K196E mutation as a genetic risk factor for deep vein thrombosis in Japanese patients. Blood. 2006;107(4):1737–8. doi:10.1182/blood-2005-09-3892.
- Miyata T, Okada H. Abnormality in blood coagulation because of protein S-K196E mutation. Brain Nerves. 2008;60:1285–93.
- Kemkes-Matthes B. Acquired protein S deficiency. Clin Investig. 1992;70(6):529–34. doi:10.1007/BF00210237.
- Ichinose M, Sasagawa N, Chiba T, Toyama K, Kayamori Y, Kang D. Protein C and protein S deficiencies may be related to survival among hemodialysis patients. BMC Nephrol. 2019;20(1):191. doi:10.1186/s12882-019-1344-8.
- van Ommen CH, Fijnvandraat K, Vulsma T, Delemarre-van de Waal HA, Peters M. Acquired protein S deficiency caused by estrogen treatment of tall stature. J Pediatr. 1999;135(4):477–81. doi:10.1016/S0022-3476(99)70171-X.
- Lalan DM, Jassawalla MJ, Bhalerao SA. Successful pregnancy outcome in a case of protein s deficiency. J Obstet Gynaecol India. 2012;62(Suppl 1):21–2. doi:10.1007/s13224-013-0363-9.
- Cui C, Yang S, Zhang J, Wang G, Huang S, Li A, Zhang Y, Qiao R. Trimester-specific coagulation and anticoagulation reference intervals for healthy pregnancy. Thromb Res. 2017;156:82–6. doi:10.1016/j.thromres.2017.05.021.
- Francis RB Jr. Protein S deficiency in sickle cell anemia. J Lab Clin Med. 1988;111:571–6.
- Pilli VS, Datta A, Afreen S, Catalano D, Szabo G, Majumder R. Hypoxia downregulates protein S expression. Blood. 2018;132(4):452–5. doi:10.1182/blood-2018-04-841585.
- D’Angelo A, Valle PD, Crippa L, Pattarini E, Grimaldi L, D’Angelo SV. Brief report: autoimmune protein S deficiency in a boy with severe thromboembolic disease. N Engl J Med. 1993;328(24):1753–7. doi:10.1056/NEJM199306173282405.
- Morange PE, Alessi MC, Barthet MC, Aillaud MF, Harlé JR, Piquet P, Juhan-Vague I. Acquired protein S deficiency, likely due to anti-PS autoantibodies, following a thrombotic event in a patient with a systemic lupus erythematosus. Thromb Haemost. 1997;78(5):1416–17. doi:10.1055/s-0038-1665422.
- Lipe B, Ornstein DL. Deficiencies of natural anticoagulants, protein C, protein S, and antithrombin. Circulation. 2011;124(14):e365–8. doi:10.1161/CIRCULATIONAHA.111.044412.
- Wigle P, Hein B, Bernheisel CR. Anticoagulation: updated guidelines for outpatient management. Am Fam Physician. 2019;100:426–34.
- Campello E, Spiezia L, Simion C, Tormene D, Camporese G, Dalla Valle F, Poretto A, Bulato C, Gavasso S, Radu CM, et al. Direct oral anticoagulants in patients with inherited thrombophilia and venous thromboembolism: a prospective cohort study. J Am Heart Assoc. 2020;9(23):e018917. doi:10.1161/JAHA.120.018917.
- Rezende SM, Simmonds RE, Lane DA. Coagulation, inflammation, and apoptosis: different roles for protein S and the protein S-C4b binding protein complex. Blood. 2004;103(4):1192–201. doi:10.1182/blood-2003-05-1551.
- Brunet D, Barthet MC, Morange PE, Alessi MC, Borgel D, Gandrille S, Aillaud MF, Juhan-Vague I. Protein S deficiency: different biological phenotypes according to the assays used. Thromb Haemost. 1998;79:446–7.
- Serra J, Sales M, Chitolie A, Domènech P, Rossi E, Borrell M, Dahlbäck B. Multicentre evaluation of IL test™ free PS: a fully automated assay to quantify free protein S. Thromb Haemost. 2002;88(6):975–83. doi:10.1055/s-0037-1613343.
- Persson KE, Hillarp A, Dahlback B. Analytical considerations for free protein S assays in protein S deficiency. Thromb Haemost. 2001;86(5):1144–7. doi:10.1055/s-0037-1616042.
- Hackeng TM, Fernández JA, Dawson PE, Kent SB, Griffin JH. Chemical synthesis and spontaneous folding of a multidomain protein: anticoagulant microprotein S. Proc Natl Acad Sci USA. 2000;97(26):14074–8. doi:10.1073/pnas.260239797.
- Tripodi A, Asti D, Chantarangkul V, Biguzzi E, Mannucci PM. Interference of factor V Leiden on protein S activity: evaluation of a new prothrombin time-based assay. Blood Coagul Fibrinolysis. 2007;18(6):543–6. doi:10.1097/MBC.0b013e328201ca8a.
- Alshaikh NA, Rosing J, Thomassen MCLGD, Castoldi E, Simioni P, Hackeng TM. New functional assays to selectively quantify the activated protein C- and tissue factor pathway inhibitor-cofactor activities of protein S in plasma. J Thromb Haemost. 2017;15(5):950–60. doi:10.1111/jth.13657.
- Neupane S, Pudasaini P, Dhakal B, Awal S, Thapa S, Subedi B. Protein S deficiency with recurrent deep vein thrombosis and post thrombotic syndrome: a case report. JNMA J Nepal Med Assoc. 2022;60(254):892–4. doi:10.31729/jnma.7694.
- Andersen BD, Bisgaard ML, Lind B, Philips M, Villoutreix B, Thorsen S. Characterization and structural impact of five novel PROS1 mutations in eleven protein S-deficient families. Thromb Haemost. 2001;86(6):1392–9. doi:10.1055/s-0037-1616741.
- Klostermeier UC, Limperger V, Kenet G, Kurnik K, Gelas MA, Finckh U, Junker R, Heller C, Zieger B, Knöfler R, et al. Role of protein S deficiency in children with venous thromboembolism. An observational international cohort study. Thromb Haemost. 2015;113(2):426–33. doi:10.1160/TH14-06-0533.
- Gupta A, Ahmed RP, Bhattacharyya M, Kannan M, Biswas A, Kalra V, Saxena R. Protein S deficiency. In: StatPearls. FL: Treasure Island; 2019. p. 233–37.
- Lou J, Yin L, Ke X, Zhang L, Xu F, Liu Z. A case-report of two patients with hereditary protein S deficiency treated by rivaroxaban. Blood Coagul Fibrinolysis. 2020;31(6):405–9. doi:10.1097/MBC.0000000000000929.
- Folkeringa N, Brouwer JLP, Korteweg FJ, Veeger NJGM, Erwich JJHM, Holm JP, Van Der Meer J. Reduction of high fetal loss rate by anticoagulant treatment during pregnancy in antithrombin, protein C or protein S deficient women. Br J Haematol. 2007;136(4):656–61. doi:10.1111/j.1365-2141.2006.06480.x.
- Peraramelli S, Thomassen S, Heinzmann A, Rosing J, Hackeng TM, Hartmann R, Scheiflinger F, Dockal M. Inhibition of tissue factor: factor VIIa–catalyzed factor IX and factor X activation by TFPI and TFPI constructs. J Thromb Haemost. 2014;12(11):1826–37. doi:10.1111/jth.12713.

