ABSTRACT
Shenling Baizhu Powder is a traditional Chinese medicine formula that has been used for thousands of years to invigorate spleen, nourish stomach and regulate immune function. The multi-herbal extracts, an improved formular from Shenling Baizhu Powder, were used in our study to investigate its immune-enhancing effects and underling mechanisms. Based on UPLC-Q-TOF-MS and SPME-GC-MS analysis, 106 chemical compounds were identified from the extracts. In vitro assays indicated that the extracts could promote cell proliferation and NO secretion of RAW264.7 cells. RNA-seq and RT-qPCR showed that RAW264.7 cells treated with extracts increased the expression of pro-inflammatory factors IL-1β, iNOS, TNF-α, and TGF-β. Western-Blot revealed that the extracts could significantly enhance ERK phosphorylation level, and slightly increased JNK and p38 phosphorylation levels. These data suggest that multi-herbal extracts have a potential of regulating immune response via the JNK/p38/ERK signalling of MAPK pathways in RAW264.7 cells.
GRAPHICAL ABSTRACT
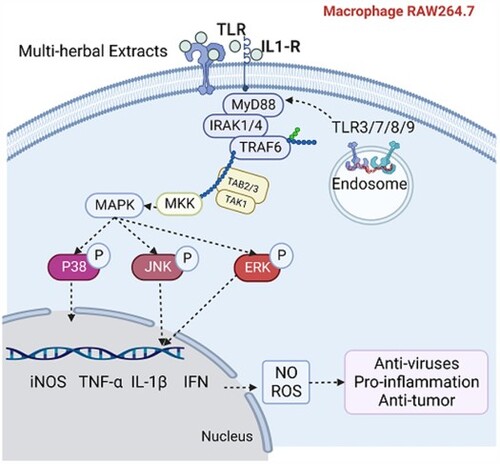
1 Introduction
The pandemic of coronavirus disease-2019 (COVID-19) wreaked devastation on the economic and quality of life globally. Since COVID-19 could cause vulnerable population very sick and even kill them, efficient strategies are urgently needed to stop the spread of the viruses and eliminate the serve post-COVID symptoms. Besides vaccination, which can provide memory responses to protect vulnerable population from severe illness, people are also actively searching for more available and effective pharmaceuticals. Traditional Chinese Medicine (TCM) is an ancient medical system that has evolved from China over thousands of years. TCM has been documented successfully controlling and treating infectious diseases (Luo et al., Citation2020). Recently, TCM also showed beneficial effects on the prevention and treatment of COVID-19 (Dai et al., Citation2020).
The immune system mounts responses against invading viral pathogens through a fast innate response and a slower but more antigen-specific adaptive response. Macrophages play an important role in innate response to viruses (Cyprian et al., Citation2021; Wu et al., Citation2020). When the host is confronted with external invaders or internal threats, macrophages as antigen-presenting cells recognize viruses, release pro-inflammatory cytokines, engulf and then present antigen peptides to T cells to activate adaptive immune response (Wang, et al., Citation2020). Besides removing foreign pathogens, activated macrophages can also aid in eliminating cancer cells (Wang, et al., Citation2020). Since macrophages play multiple roles in the immune response, they are frequently used as an ideal cell model to assess the immunomodulatory properties of bioactive compounds (Wu et al., Citation2020).
During the pandemic of COVID-19, TCM has been recommended to prevent and ameliorate the illness of COVID-19 from different stages (Lyu et al., Citation2021). Clinical data showed that in the combined therapy with western medicine, TCM showed beneficial outcomes in treating COVID-19 (Lyu et al., Citation2021). With low toxicity, minimal side effects, and fast absorption, herbal materials of medicine food homology are gaining attention and becoming unique dietary ingredients in China (Liu et al., Citation2016). Shenling Baizhu powder, a formulation of numerous natural herbal ingredients, is first mentioned in the Song Dynasty’s “Taiping Huimin Hejiju prescription.” This formulation is considered as the representative prescription for the treatment of spleen and stomach disorders, qi deficiency, and diarrhoea (Wang, et al., Citation2020). The addition and subtraction of Shenling Baizhu powder has been widely used in the treatment of numerous disorders, including diarrhoea (Zhao & Cao, Citation2019), ulcerative colitis (Zhang, Citation2017), and bronchial asthma (Xu et al., Citation2020). Previous studies have shown that Shenling Baizhu powder has the potential to boost immune response and promote the expression of anti-inflammatory factors (Zhang et al., Citation2018).
In our study, we used an herbaceous compound prescription (HCP), which is a formular of diet therapy. The integrant of this HCP is modified from Shenling Baizhu powder, containing Panax Ginseng, Poria cocos, Fried yam, Angelica sinensis, Tangerine peel, Fried malt, Fried coix seed, Fried Galli Gigerii endothelium corneum, Lotus seeds, Fried hawthorn berry, Agastache rugosa, Pueraria lobata, seeds of Alpiniae oxy-phyllae Fructus, Amomum villosum and Zingiber. The integrant of the HCP follows the rules of monarch-minister-assistant envoy, which is a basic principle of prescription compatibility used in TCM. The prescriptions have the properties of invigorating spleen, nourishing stomach and aiding digestion (Wang, Luo, et al., Citation2020). However, the mechanism effect of this HCP on immune response is not clear. Therefore, in this study we investigated the chemical composition and the immunoregulatory effects of this HCP on mouse macrophages RAW264.7 cells. Firstly, ACQUITY UPLC I-class ultra-performance liquid chromatography, VION ion mobility quadrupole time-of-flight mass spectrometer (UPLC-Q-TOF-MS), and solid-phase microextraction gas chromatography/mass spectrometry (SPME-GC-MS) were used to analyse the bioactive compounds of the extracts. Then, the cell viability and the production of nitric oxide (NO) were measured in RAW264.7 cells in vitro. Lastly, the mechanisms of immunomodulatory activities were analysed and confirmed by RNA sequencing (RNA-seq), real-time quantitative polymerase chain reaction (RT-qPCR), and Western Blot. This study sheds insight into the immunomodulatory properties of the extracts and provides evidence to the clinical application of the extracts as a dietary supplement for treating infectious disease and potential cancer.
2 Materials and methods
2.1. Materials and chemicals
The extracts were supplied by Xuncaofang (Shenyang) Biotech company (batch number 20200509). The concentration of the extracts is 0.3 g/mL. The storage time of the extracts is 12 months. The extracts consist of Panax Ginseng (PG, from Panax ginseng), Poria cocos (PC, from Poria cocos), Fried yam (FY, from Dioscorea oppositifolia), Angelica sinensis (AS, from Angelica sinensis), Tangerine peel (TP, from Citrus reticulata), Fried malt (FM, from Hordeurn vulgare), Fried coix seed (FCS, from Coix lacryma-jobi), Fried Galli Gigerii endothelium corneum (FGGEC, from Gallus gallusdomesticus), Lotus seeds (LS, from Nelumbo nucifera), Fried hawthorn berry (FHB, Crataegus pinnatifida), Agastache rugosa (AR, Agastache rugosa), Pueraria lobata (PL, Pueraria lobata), seeds of Alpiniae oxy-phyllae Fructus (AOM, from Alpinia oxyphylla), Amomum villosum (AVL, from Amomum villosum) and Zingiber (ZI, from Zingiber odoriferum).
2.2. UPLC-Q-TOF-MS analysis of small-molecule compounds
To ensure the reliability of peak identification, a database of the extracts was established based on integrating the human metabolome database (HMDB, https://hmdb.ca), Chemical Book (https://www.chemicalbook.com/ProductIndex.aspx), National Library of Medicine (https://pubchem.ncbi.nlm.nih.gov/) and related literature. Furthermore, a chemical compound library was created, which includes the compound name, molecular formula, structural diagram.
The small molecules of the extracts were identified by UPLC-Q-TOF-MS. The UPLC-Q-TOF-MS analysis was implemented on an ACQUITY UPLC I-class system (Waters, MA, USA) coupled with Vion IMS QTOF Mass Spectrometer. The column (BEH C18 1.7 µm, 2.1 × 100 mm) was used to separate the extracts at 45°C with the flow rate of 0.4 mL/min. The mobile phase was composed of water (A) containing 0.1% formic acid and acetonitrile (B) containing 0.1% formic acid, with the following gradient: 0–3 min, 95% A, 5% B; 3–10 min, 80% A, 20% B; 10–12 min, 100% B; 15–19 min, 5% A, 95% B. The injection volume was 1 μL.
The ion mode used was electrospray ionization (ESI). The electrospray ionization conditions were set as follows: capillary voltage, 2 kV (negative); cone voltage, 40 V; desolvation temperature, 450°C; desolation gas, 900 L/h; cone gas, 50 L/h; source temperature, 115°C; acquisition range, 50–1000 m/z; scan rate, 0.2 s; collision energy, 6 eV.
2.3. SPME-GC-MS analysis of volatile compounds
SPME-GC-MS was used to identify the volatile substances of the extracts. The volatile components were extracted using SPME, a highly sensitive volatile extraction technique. After that, the extracted volatiles were examined using Agilent 7890 gas chromatograph (Agilent, Palo Alto, CA, USA) equipped with an MSD 5973 Mass Spectrometer (Agilent, Palo Alto, CA, USA).
The extracts were poured into a 15 mL headspace bottle, and the bottle was incubated at 50°C for 15 min. After that, a 50/30 μm DVB/CAR/PDMS (2 cm, PA, USA) extraction fibre was inserted into the headspace bottle by an annual solid-phase microextraction device for extraction for 30 min. Then, the extraction fibre was taken out and inserted into the injection port of the GC-MS immediately, and desorbed for 5 min.
The GC-MS working conditions were as follows: the carrier gas was high purity helium (99.999%) with a constant flow rate of 1 mL/min, the inlet temperature was 260°C, splitless, the initial temperature of column (30 m × 250 μm × 0.25 μm, Agilent 122-7032: WAX DB-WAX, Palo Alto, CA, USA) maintained at 40°C for 5 min, heated to 220°C at a rate of 5°C/min, heated to 250°C at a rate of 20°C/min and hold for 2.5 min; The mass spectrometer was operated in the electron impact of 70 eV, scanning the range of 20/400 m/z in full scan acquisition mode. The mass spectra and retention time of each GC peak in the total ion chromatography were compared with the data system library (NIST 2014 MS Library).
2.4. Cell culture
RAW264.7 cells were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). The cells grew in DMEM (Gibco, Shanghai, China) with antibiotics 100 U/mL penicillin, 100 mg/mL streptomycin (Gibco, Rockville, MD), and 10% FBS (HyClone, GE, USA). Cells were incubated at 37 °C in a 5% CO2 incubator.
2.5. Cell viability assay
RAW267 cells were inoculated in a 96-well microplate for 24 h, followed by treated with different concentration of the extracts (1, 2.5, 5 mg/ml) for another 24 h (Liu et al., Citation2021). PBS and lipopolysaccharide (LPS, 1 μg/mL) were employed as controls. Then, 10 μL of CCK-8 solution (Sangon Biotech, Shanghai, China) was added to each well and incubated for 0.5 h. Absorbance values were estimated at 450 nm by a microplate reader (BIO-TEK®, VT, USA).
2.6. Determination of nitric oxide (NO)
The level of NO production was measured by a nitric oxide assay kit (Beyotime Institute of Biotechnology, China). RAW 264.7 cells were first inoculated in a 96-well microplate, and then treated with different concentrations of the extracts (1, 2.5, 5 mg/mL). The NO concentration was calculated using sodium nitrite (NaNO2) as a standard curve, according to the manufacturer’s methodology.
2.7. Analysis of immune-associated gene expression
The mRNA levels of iNOS, TNF-α, IL-1β, and TGF-β were analysed by RT-qPCR (Zhang et al., Citation2018). The concentration of RNA was measured, followed by reverse transcription into cDNA. presents the RT-qPCR primer sequences.
Table 1. RT-qPCR Primers.
2.8. RNA sequencing
After the treatment with PBS and the extracts at different concentrations (1, 2.5, 5 mg/mL), the total RNA was extracted using RNAprep Pure Cell/Bacteria Kit (Tiangen, Beijing, China), and sent to the BioMarker technologies company for quality control and complementary DNA (cDNA) library construction. Trimmomatic was used to trim low quality reads and remove adapters of raw data. The hisat2 was used to align the trimmed sequence to the mouse genome (GRCm39). The differentially expressed genes (DEGs) were analysed using DESeq2 software (version 1.32.0). The false discovery rate was P value < 0.05 and |log2FoldChange| > 1. R software was used to conduct cluster analysis and GO enrichment analysis. The core analysis, biological function, and comparison analysis were detected by IPA software. In the following analysis, the groups of extracts treatment of 1, 2.5 and 5 mg/mL were separately named as Dose 1, Dose 2.5 and Dose 5.
2.9. Analysis of protein-associated MAPK pathways
Western Blot was employed to analyse the protein-associated MAPK pathways (Liu et al., Citation2021). After 1 h treatment, the total protein was extracted with lysis buffer (RIPA: PMSF = 100:1). The extracted proteins were then separated by 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) gels and transferred to 0.22 μm microporous polyvinylidene fluoride membranes (Sangon Biotech, Shanghai, China). After being blocked with 5% skim milk for 2 h, the membrane was incubated with the primary antibodies (Table A1) at 4°C overnight, and then incubated with the secondary antibodies for 2 h. Finally, the proteins were detected with Enhanced ECL Chemiluminescent Substrate Kit Western Blot analysis substrate (Affinity Biosciences, OH, USA).
2.10. Statistical analysis
Data were presented as the mean ± standard deviation (SD). Graph Pad Prism 8 software was used for statistical analysis. All experiments were repeated three or six times, and significance was indicated as ∗p <0 .05; ∗∗p < 0.01; ∗∗∗p <0.001; and ∗∗∗∗p <0 .0001.
3. Results
3.1. Chemical compounds of the extracts
To identify chemical compounds in the extracts of Shenling Baizhu Powder, a chemical compound library containing 466 compounds was conducted (data not shown). Based on the library, UPLC-Q-TOF-MS analysis ((a,c)) found 45 small molecule compounds including 18 flavonoids, 14 saponins, 7 phenolic acids, 2 sugars, 2 amino acids, 1 quinone and 1 triterpene (Table S2). SPME-GC-MS analysis ((b,d)) showed 61 volatile compounds consisting of 19 aromatic compounds, 15 monoterpenes, 6 aldehydes, 5 alcohols, 5 ketones, 4 esters, 3 acids, 2 heterocyclic compounds and 2 hydrocarbons (Table S3). Collectively, a total of 106 chemical compounds were identified in the extracts.
Figure 1. The chemical compounds in the extracts. (a) The total iron chromatograms in negative ion mode of UPLC-Q-TOF-MS analysis. (b) The total iron chromatogram in the extracts of SPME-GC-MS analysis. (c) The small-molecules. (d) The volatile compounds. SPME-GC-MS: solid-phase microextraction gas chromatography/mass spectrometry; UPLC-Q-TOF-MS: ACQUITY UPLC I-class ultra-performance liquid chromatography, VION ion mobility quadrupole time-of-flight mass spectrometer.

3.2. Herbal extracts enhanced RAW267 cell viability in a dose-dependent manner
After treated with the extracts (1–5 mg/mL), the viability of RAW264.7 cells was increased in a dose-dependent manner (). The cell viability reached a maximum of 150.167 (p ≤0.001) at 5 mg/mL of the extracts, remaining lower than that of the LPS group.
Figure 2. Effects of the extracts on the viability of macrophage RAW264.7 cells. Control cells were treated with PBS only, and LPS cells were treated with 1 μg/mL of LPS as a positive control. Data were expressed as the mean ± SD (n = 5); *p ≤0 .05; ∗∗p ≤0 .01; ∗∗∗p ≤ 0.001 compared with the control group. SD: standard deviation; ConA: concanavalin A; NO: nitric oxide.

3.3. Herbal extracts increased DEGs related to cytokine-mediated pathways
There were 2159 DEGs identified in the Dose 1 compared to control group, of which 1056 genes were up-regulated ((a)) and 1139 genes were down-regulated ((a,b)). There were 3007 DEGs found in the Dose 2.5 compared to control group, of which 1534 were up-regulated ((a)) and 1473 were down-regulated ((a,b)). In higher dose group (Dose 5), there were 1591 up-regulated DEGs ((a)) and 1477 down-regulated DEGs (total 3068 DEGs) were identified ((a,b)). The Principal Component Analysis (PCA) diagram and hierarchical clustering showed the distinguish gene clusters between the treatment of the extracts and control ((c,d)).
Figure 3. General analysis of RNA-seq data. (a) Venn diagram of up-regulated DEGs. (b) Venn diagram of down-regulated DEGs. (c) PCA diagram of DEGs in the three treatments between the control. (d) Hierarchical clustering heat map of DEGs in the three treatments between the control. DEGs: differentially expressed genes. PCA: Principal Component Analysis.

GO enrichment analysis of DEGs between the three doses treatment groups and the control group showed that the top three signalling pathways were cytokine-mediated signalling pathway, NF-κB signalling pathway, and cytoplasmic pattern recognition receptor signalling pathway in response to virus ((a)). The pathway enrichment of Ingenuity Pathway Analysis (IPA) showed that the top three signalling pathways were the role of hypercytokinemia/hyperchemokinemia in the pathogenesis of influenza, IL-17 signalling, and TREM1 signalling ((b)). Disease and function analysis ((c)) suggested that the DEGs identified in the treatment groups were engaged in the recruitment and activation of immune cells, the replication of viruses, chemotaxis and expansion of cells.
Figure 4. Function analysis of DEGs. (a) GO enrichment analysis of the DEGs in the three treatments between the control. (b) Canonical pathway analysis of DEGs in IPA software. (c) Disease and function analysis of DEGs in IPA software. DEG: differentially expressed genes; IPA: Ingenuity Pathway Analysis.

3.4. Herbal extracts increased NO and inflammatory cytokines production
In response to inflammatory signals, immune myeloid cells such as macrophages can produce cytokines and NO, which are critical for pathogens killings (Palmieri et al., Citation2020). Therefore, in order to test the immune-regulatory effects of the extracts, we measured the level of NO and pivotal cytokines production. We also included LPS as a positive control. We found an increased NO production in the range of 1∼5 mg/mL compared to the control group. NO production increased with the treatment of the extracts and reached a maximum at 5 mg/mL of the extracts ((a)). Results of RT-qPCR analysis of four DEGs genes showed that the extracts significantly increased the expression levels of iNOS ((b); p ≤ 0.001) and IL-1β ((c); p ≤0.001), compared with the control group. The mRNA level of TNF-α ((d); p ≤0.001) also increased and reached the maximum at 2.5 mg/mL of the extracts (p ≤0.001). The mRNA level of anti-inflammatory cytokines TGF-β ((e); p ≤ 0.001) decreased.
Figure 5. Effects of the extracts on the expression of inflammatory mediators in RAW264.7 cells. Cells were treated with different concentrations of extracts (1, 2.5 and 5 mg/mL) for 6 h. The expression level of NO(a) was measured by a nitric oxide assay kit. The mRNA expression of iNOS (b), IL-1β(c), TNF-α (d), TGF-β1 (e), was measured by RT-qPCR. Data were expressed as the mean ± SD (n = 3). *p ≤0 .05; ∗∗∗p ≤0.001 versus 0 mg/mL group. SD: standard deviation; iNOS: inducible nitric oxide synthase; TNF-α: tumour necrosis factor; IL: interleukin; TGF-β1: transforming growth factor-β1; messenger RNA: mRNA.

3.5. Herbal extracts stimulated cells via protein-associated MAPK pathways
Results of Western Blot analysis showed that the extracts stimulated the phosphorylation of ERK1/2, JNK, and p38 in the MAPK pathways (). The phosphorylation of p-ERK/ERK increased obviously in the range of 1–5 mg/mL. The p-JNK/JNK and p-p38/p38 also increased slightly with the treatment with the extracts ().
Figure 6. Effect of the extracts on proteins associated with the MAPK pathways. RAW264.7 cells were treated with 0, 1, 2.5, 5 mg/mL extracts for 1 h, and then the total protein in the cells was extracted by Western Blot's method. Data were expressed as the mean ± SD (n = 3). ∗∗p ≤0.01 versus 0 group. MAPK: mitogen-activated protein kinase; SD: standard deviation.

4. Discussion
The outbreak of COVID-19 has brought serious influence to human production and life. Vaccination is the primary way to equip the immune system to fight virus invasion, however, searching for immunomodulatory agents as adjuvants to boost the immune system is also important. One way is to discover new food therapy from traditional Chinese diet therapy prescriptions. Many traditional Chinese medicines have been proved could boost immune response (Fan et al., Citation2020). Many natural products in China have been applied as potential immunomodulatory agents to tackle the disease (Razmi et al., Citation2020). The extracts used in our study, a food therapy formula, were modified from a classic clinical prescription “Shenling Baizhu powder.” This study provides a useful reference for the exploration of dietary therapy formulas from ancient Chinese prescriptions and the evaluation of prescriptions. At the same time, it also plays a positive role in promoting the development of the traditional Chinese medicine industry. In the study, we characterized and classified the chemical composition of the extracts to reveal the material basis for the pharmacological action of the extracts. Moreover, we found that the extracts were rich in flavonoids, phenols, saponins, aldehydes.
Macrophage, with the functions of organ development, disease prevention, and tissue restitution, is an integral part of the immune system (Horst et al., Citation2019; Yu et al., Citation2021). Therefore, murine macrophage RAW264.7 cell line was used as an in vitro cell model to investigate the immunomodulatory effect of the extracts (Wu et al., Citation2020). In the present study, RAW264.7 cells were employed to evaluate the immunomodulatory effect of extracts on cell proliferation, iNOS gene expression and NO production. Cell proliferation is the process that results in an increased number of cells, and is defined by a balance between cell divisions and cell loss through cell death or differentiation. Our data suggested that the extracts could enhance the proliferation of RAW264.7 cells in vitro (). Meanwhile, both iNOS gene expression and NO production were significantly up-regulated by the extracts ((a,b)). It is known that activation of macrophage release iNOS which triggers the synthesis and secretion of NO (Ji et al., Citation2019). In addition, the secretion of NO help in removing pathogens, regulating adaptive immunity, and regulating energy metabolism (Anavi & Tirosh, Citation2020). Overall, the up-regulation of the expression of iNOS and NO in cells treated with the extracts indicated that the extracts could activate macrophage RAW264.7 cells.
In recent years, with the advent of next-generation sequencing along with the development of various bioinformatics tools, RNA-Seq-based transcriptome analysis has become much more widespread in the field of biological research. Using this technique, researchers explore the transcriptome of non-model organisms for which a reference genome is not available. This has especially made human health researchers march towards this technology to understand pathogenic processes and immune reactions in human life during the event of infection (Sudhaga et al., Citation2018).
In this work, RNA-seq analysis found that the gene expressions of TNF, IL-1β, and TGF-β1 were significantly increased from the extracts-treated macrophage (Table A4). TNF-α plays a vital role in the immune response by regulating multiple signalling pathways (Holbrook et al., Citation2019). IL-1β could further boost the expression of other pro-inflammatory mediators, such as reactive oxygen species, NO, and cyclocase-2, etc., and plays a key role in the acute inflammatory response (Gupta & Barthwal, Citation2018). In addition, TGF-β1 is a crucial anti-inflammatory regulator, which could alleviate the immune response to pathogens (Zhang et al., Citation2016). RT–PCR showed that the extracts increased the expression of TNF-α, IL-1β and reduced the expression of TGF-β in the RAW264.7 cells ((c,d,e)), which is consistent with the data from RNA-sequencing. Numerous studies have shown that M1 macrophages function in the process of pathogen removal by promoting the synthesis of pro-inflammatory mediators (e.g. TNF-α, IL-1β, reactive oxygen and nitrogen species) (Zhang & Wang, Citation2014). These results indicated that the extracts might display significant immunomodulatory activities by induced M1 macrophage phenotype.
IL-17, a powerful pro-inflammatory cytokine, is fundamental in a diversity of processes including host defense, tissue repair, inflammatory disease pathogenesis and cancer progression (Li et al., Citation2019). Consistent with TLR/IL-1R cytokine homology, IL-17 signalling promotes inflammatory response through NF-κB-induced gene expression and activation of MAPK pathways (Amatya et al., Citation2017). MAPK signalling pathways includes JNK, ERK1/2, p38, and ERK5 signalling pathways (Gehart et al., Citation2010). Among the MAPK pathways, ERK1/2 is predominantly engaged in cell growth control, whereas the JNK and p38 signalling pathways are primarily involved in some cellular responses, including survival, apoptosis, and inflammation (Amatya et al., Citation2017). The p38 signalling way was discovered to be differentially expressed and participated in the immune response throughout the network (). Meanwhile, upstream analysis (Table S4) revealed that the expression of JNK and ERK was also up-regulated in a dose-dependent manner. Our Western Blot results () showed that the ERK signalling pathway was remarkably activated, and the P38 and JNK signalling pathways were slightly up-regulated, indicating that the MAPK signalling pathways were activated. The previous studies considered that the activation of MAPK signalling pathway would initiate the immune response by promoting the secretion of pro-inflammatory mediators, such as iNOS, IL-1β, TNF-α, NO, and inhibiting the anti-inflammatory cytokines, such as TGF-β (Liu et al., Citation2021; Zusso et al., Citation2019), which substantiated our results.
5. Conclusion
The extracts contained rich bioactive substances including flavonoids, phenols, saponins, aldehydes, etc., and possessed immunostimulatory effects on RAW264.7 cells, such as promoting cell growth, and pro-inflammatory mediators release. In vitro assays revealed that MAPK signalling pathways might be a plausible mechanism for the immunomodulatory effects of the extracts. These findings suggested that the extracts could be applied as a potential immunomodulatory agent, as well as a good benchmark for assessing dietary supplements.
Sample availability
Samples of the “Shenling Baizhu Powder” are available from the authors.
Supplemental Material
Download Zip (184.4 KB)Acknowledgments
The authors would like to acknowledge the help from Instrumental Analysis Center of Shanghai Jiao Tong University.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data presented in the study are available on request from the corresponding author.
Additional information
Funding
References
- Amatya, N., Garg, A. V., & Gaffen, S. L. (2017). IL-17 signaling: The yin and the yang. Trends in Immunology, 38(5), 310–322. https://doi.org/10.1016/j.it.2017.01.006
- Anavi, S., & Tirosh, O. (2020). iNOS as a metabolic enzyme under stress conditions. Free Radical Biology and Medicine, 146(2020), 16–35. https://doi.org/10.1016/j.freeradbiomed.2019.10.411
- Cyprian, F., Sohail, M. U., Abdelhafez, I., Salman, S., Attique, Z., Kamareddine, L., & Al-Asmakh, M. (2021). SARS-CoV-2 and immune-microbiome interactions: Lessons from respiratory viral infections. International Journal of Infectious Diseases, 105(2021), 540–550. https://doi.org/10.1016/j.ijid.2021.02.071
- Dai, Y. J., Wan, S. Y., Gong, S. S., Liu, J. C., Li, F., & Kou, J. P. (2020). Recent advances of traditional Chinese medicine on the prevention and treatment of COVID-19. Chinese Journal of Natural Medicines, 18(12), 881–889. https://doi.org/10.1016/S1875-5364(20)60031-0
- Fan, K. J., Li, Y. W., Wu, J., Li, J., Zhang, J., Wang, Q. S., Xu, B. X., Cai, Q., & Wang, T. Y. (2020). The traditional Chinese medicine fufang shatai heji (STHJ) enhances immune function in cyclophosphamide-treated mice. Evidence-Based Complementary and Alternative Medicine, 2020, 3849847. https://doi.org/10.1155/2020/3849847
- Gehart, H., Kumpf, S., Ittner, A., & Ricci, R. (2010). MAPK signalling in cellular metabolism: Stress or wellness? EMBO Reports, 11(11), 834–840. https://doi.org/10.1038/embor.2010.160
- Gupta, P., & Barthwal, M. K. (2018). IL-1 β genesis: The art of regulating the regulator. Cellular & Molecular Immunology, 15(11), 998–1000. https://doi.org/10.1038/s41423-018-0054-7
- Holbrook, J., Lara-Reyna, S., Jarosz-Griffiths, H., & McDermott, M. F. (2019). Tumour necrosis factor signalling in health and disease. F1000Research, 8(111), 111. https://doi.org/10.12688/f1000research.17023.1
- Horst, A. K., Tiegs, G., & Diehl, L. (2019). Contribution of macrophage efferocytosis to liver homeostasis and disease. Frontiers in Immunology, 10(2019), 2670. https://doi.org/10.3389/fimmu.2019.02670
- Ji, L., Zhao, X., Zhang, B., Kang, L., Song, W., Zhao, B., Xie, W., Chen, L., & Hu, X. (2019). Slc6a8-Mediated creatine uptake and accumulation reprogram macrophage polarization via regulating cytokine responses. Immunity, 51(2), 272–284.e7. https://doi.org/10.1016/j.immuni.2019.06.007
- Li, X., Bechara, R., Zhao, J., McGeachy, M. J., & Gaffen, S. L. (2019). IL-17 receptor-based signaling and implications for disease. Nature Immunology, 20(12), 1594–1602. https://doi.org/10.1038/s41590-019-0514-y
- Liu, W. J., Liu, D. B., Zhang, Z. X., & Qin, D. (2016). Research progress on antibacterial and anti-inflammatory effects of medicinal and edible plants. Chin Tradit Herb Drugs, 47(24), 3535–3542. https://doi.org/10.7501/j.issn.0253-2670.2016.19.029
- Liu, Y., Li, Q. Z., Li, L. D., & Zhou, X. W. (2021). Immunostimulatory effects of the intracellular polysaccharides isolated from liquid culture of ophiocordyceps sinensis (ascomycetes) on RAW264.7 cells via the MAPK and PI3K/Akt signaling pathways. Journal of Ethnopharmacology, 275, 114130. https://doi.org/10.1016/j.jep.2021.114130
- Luo, H., Tang, Q. L., Shang, Y. X., Liang, S. B., Yang, M., Robinson, N., & Liu, J. P. (2020). Can Chinese medicine be used for prevention of corona virus disease 2019 (COVID-19)? A review of historical classics, research evidence and current prevention programs. Chinese Journal of Integrative Medicine, 26(4), 243–250. https://doi.org/10.1007/s11655-020-3192-6
- Lyu, M., Fan, G. W., Xiao, G. X., Wang, T. Y., Xu, D., Gao, J., Ge, S. Q., Li, Q. L., Ma, Y. L., Zhang, H., Wang, J. G., Cui, Y. L., Zhang, J. H., Zhu, Y., & BL, Z. (2021). Traditional Chinese medicine in COVID-19. Acta Pharmaceutica Sinica B, 11(11), 3337–3363. https://doi.org/10.1016/j.apsb.2021.09.008
- Palmieri, E. M., McGinity, C., Wink, D. A., & McVicar, D. W. (2020). Nitric oxide in macrophage immunometabolism: Hiding in plain sight. Metabolites, 10(11), 429. https://doi.org/10.3390/metabo10110429
- Razmi, M., Hashemi, F., Gheytanchi, E., Dehghan, Manshadi, M., Ghods, R., & Madjd, Z. (2020). Immunomodulatory-based therapy as a potential promising treatment strategy against severe COVID-19 patients: A systematic review. International Immunopharmacology, 88, 106942. https://doi.org/10.1016/j.intimp.2020.106942
- Sudhaga, A., Kumar, G., & El-Matbouli, M. (2018). Transcriptome analysis based on RNA-Seq in understanding pathogenic mechanisms of diseases and the immune system of fish: A comprehensive review. International Journal of Molecular Sciences, 19(1), 245. https://doi.org/10.3390/ijms19010245
- Wang, C., Luo, J. P., Huang, Q. S., Zhang, C. T., Dong, X. W., & Q, C. (2020). Research progress of shenlingbaizhu powder in treating respiratory system diseases. Journal of Traditional Chinese medicine, 32(6), 1178–1182. https://doi.org/10.16448/j.cjtcm.2020.0649
- Wang, J., Fang, X. B., Wu, T., Fang, L., Liu, C. L., & Min, W. H. (2020). In vitro immunomodulatory effects of acidic exopolysaccharide produced by Lactobacillus planetarium JLAU103 on RAW264.7 macrophages. International Journal of Biological Macromolecules, 156(2020), 1308–1315. https://doi.org/10.1016/j.ijbiomac.2019.11.169
- Wu, M. Q., Feng, H. F., Song, J. X., Chen, L. X., Xu, Z. Z., Xia, W., & Zhang, W. Q. (2020). Structural elucidation and immunomodulatory activity of a neutral polysaccharide from the Kushui Rose (Rosa setate x Rosa rugosa) waste. Carbohydrate Polymers, 232(2020), 115804. https://doi.org/10.1016/j.carbpol.2019.115804
- Xu, F., Zhao, F. S., Ma, S. H., Wang, J. R., & Wei, G. H. (2020). Research progress of clinical application of shenlingbaizhu powder by jingfang. Chin Naturopat, 28(7), 106–108. https://doi.org/10.19621/j.cnki.11-3555/r.2020.0751
- Yu, C. W., Chen, H., Du, D. H., Lv, W. T., Li, S. J., Li, D. F., Xu, Z. X., Gao, M., Hu, H. L., & Liu, D. C. (2021). β-glucan from Saccharomyces cerevisiae alleviates oxidative stress in LPS-stimulated RAW264.7 cells via Dectin-1/Nrf2/HO-1 signaling pathway. Cell Stress and Chaperones, 26(4), 629–637. https://doi.org/10.1007/s12192-021-01205-5
- Zhang, F., Wang, H. S., Wang, X. F., Jiang, G. M., Liu, H., Zhang, G., Wang, H., Fang, R., Bu, X. Z., Cai, S. H., & Du, J. (2016). TGF-β induces M2-like macrophage polarization via SNAIL-mediated suppression of a pro-inflammatory phenotype. Oncotarget, 7(32), 52294–52306. https://doi.org/10.18632/oncotarget.10561
- Zhang, L., & Wang, C. C. (2014). Inflammatory response of macrophages in infection. Hepatobiliary & Pancreatic Diseases International, 13(2), 138–152. https://doi.org/10.1016/S1499-3872(14)60024-2
- Zhang, Y. P., Tang, K. R., Deng, Y. J., Chen, R. S., Liang, S., Xie, H. J., He, Y. F., Chen, Y. N., & Yang, Q. H. (2018). Effects of shenling baizhu powder herbal formula on intestinal microbiota in high-fat diet-induced NAFLD rats. Biomedicine & Pharmacotherapy, 102(2018), 1025–1036. https://doi.org/10.1016/j.biopha.2018.03.158
- Zhang, Z. Y. (2017). Observation on treating chronic ulcerative colitis with Shenling baizhu san. Journal of Traditional Chinese medicine, 41, 65–68. https://doi.org/10.3969/j.issn.1674-7860.2017.16.047. In Chinese.
- Zhao, Y. J., & Cao, Z. Q. (2019). Clinical observation and safety evaluation of Shenlingbaizhu powder combined with pinaverium bromide tablets in the treatment of diarrhea type irratable bowel syndrome. World Chinese Medicine, 14(05), 1278–1281. https://doi.org/10.3969/j.jssn.1673-7202.2019.05.047. In Chinese
- Zusso, M., Lunardi, V., Franceschini, D., Pagetta, A., Lo, R., Stifani, S., Frigo, A. C., Giusti, P., & Moro, S. (2019). Ciprofloxacin and levofloxacin attenuate microglia inflammatory response via TLR4/NF-kB pathway. Journal of Neuroinflammation, 16(1), 148. https://doi.org/10.1186/s12974-019-1538-9
