ABSTRACT
4-coumaric acid coenzyme A ligase (4CL) plays an important role in plant growth, development and resistance. Based on the Musa acuminata (A genome) genome database, we performed genome-wide identification and evolutionary analysis of the 4CL gene family.The results showed that there were 20 members of 4CL, and the amino acids encoded ranged from 160 to 700, all of which were hydrophobic proteins. All the members of the 4CL gene family had no signal peptide, and phosphorylation sites ranged from 10 to 60. The 4CL genes were uniformly distributed and disordered in the chromosomes. All protein members can interact with each other.Transcriptome expression analysis showed that Ma4CL3-2 expression was down-regulated and Ma4CL11-1 expression was up-regulated. Musa acuminata genomics has 22 and 19 pairs of co-linear members with Musa balbisiana and Musa itinerans genomics, respectively.The above results provide a good foundation for further research on the function of the 4CL genes and resistance breeding.
Highlights
Twenty 4CL gene family members were found in banana (Musa acuminata) genomes.
Banana 4CLs were expressed in response to low temperature stress.
The members of the banana 4CL gene family were found to be relatively structurally diverse, and it was further hypothesised that 4CL may be involved in plant lignin synthesis and closely related to the process of plant differentiation into monocotyledons and dicotyledons, based on the hypothesis of Huang et al.
Abbreviations
| 4CL | = | 4-coumaric acid coenzyme A ligase |
| S | = | Syringyl |
| G | = | Guaiacyl |
| H | = | P-hydroxyphenyl |
| Arabidopsis | = | Arabidopsis thaliana |
| PI | = | Theoretical pI |
| GRAVY | = | Grand average of hydropathicity |
| CDS | = | Coding sequence |
| gDNA | = | genomic DNA |
| UTR | = | Untranslated region |
| NJ | = | Neighbourhood Joining |
Introduction
Banana (Musa nana Lour.) is a large herb of the genus Musa in the Musa family and is often affected by typhoons in the main production areas, resulting in broken and fallen stems and severe losses. 4-Coumaric acid: coenzyme A ligase (4CL) is located at the entrance of the metabolic pathway of plant lignin synthesis and regulates the synthesis of lignin monomers, which it is the rate-limiting enzyme in the process of plant lignin synthesis and plays an important role in the process of lignin synthesis (Gao et al., Citation2012; Xiang et al., Citation2013; Xiaohui et al., Citation2020). Banana stems contain high levels of lignin and cellulose. Lignin is a major component of the cell wall and plays an irreplaceable role in plant development, water transport, mechanical support and resistance to pests and diseases (Jullyana et al., Citation2010; Liu, cheng, et al., Citation2018). Natural lignin polymers generally include syringyl (S), guaiacyl (G) and p-hydroxyphenyl (H), which are each composed of three major monomers, coumary alcohol, pineal alcohol and mustard alcohol. Lignin H is synthesised using phenylalanine as the starting material, through a series of reductive reactions under the action of cinnamic acid, coumaric acid, p-coumaryl coenzyme A, coumarin, coumaryl alcohol and enzymes, while, lignin G and lignin S, which have a complex structure, are synthesised by enzymatic action using the lignin H synthesis intermediate as substrate (Behr et al., Citation2019; Xinting et al., Citation2019; Zhao, Citation2016; Zhao & Dixon, Citation2011).
4CL (4-coumaric acid: coenzyme A ligase) is the key enzyme for the synthesis of p-coumaroyl coenzyme A (the three lignin precursors) and plays a key role between the branching pathways linking the lignin precursors (Jinshan et al., Citation2011; Lavhale et al., Citation2018). 4CL catalyses the formation of the corresponding coenzyme A esters from cinnamic acid and its hydroxyl or methoxy derivatives, and these intermediates then enter the branched phenylpropane derivative synthesis pathways to produce the final lignin monomers (Hu et al., Citation1998; Moti et al., Citation2012). Huang Shengxiong et al. analysed the available plant 4CL sequences and found that the plant 4CL genes were divided into 2 main groups: the first group consisted of the 4CL genes of dicots (Arabidopsis thaliana (L.) Heynh, Glycine max (Linn.) Merr., Nicotiana tabacum Linn., Populus L., et al.); the second group consisted of the 4CL genes of monocots and gymnosperms (Oryza sativa L., Zea mays L., Lolium perenne). This suggests that the evolutionary process of plants is closely related to the 4CL genes and that the differentiation of plants into dicots and monocots occurred later than the presence of 4CL genes in plants (Shengxiong et al., Citation2008; Cao et al., Citation2015). Species currently being studied for the 4CL gene include Gerbera jamesonii Bolus (Wang et al., Citation2020), Betula alnoides Hamilt. (Li et al., Citation2021), Piper nigrum L. (Fan et al., Citation2020), Cunninghamia lanceolata (Lamb.) Hook (Gaige et al., Citation2020), Betula platyphylla Suk. (Meng et al., Citation2017), Ginkgo biloba L. (Jing et al., Citation2016), Camellia sinensis (L.) (Guo, Citation2015), Pyrus bretschneideri Rehd (Yunpeng et al., 2015), Pinus massoniana Lamb (Liu et al., Citation2013), Populus L. (Li, Citation2009), Miscanthus sinensis Anderss. (Chen, Citation2009), Gossypium hirsutum Linn. (Sun & Sun, Citation2020), Arabidopsis thaliana (L.) Heynh (Hussain et al., Citation2018), Oryza sativa L. (Jinshan et al., Citation2011), et al.
Plant lodging is a widespread problem, usually resulting in reduced photosynthesis, reduced fruit production capacity and damaged transport tissues. Improving plant resistance to lodging is therefore of great research interest (Gao, Citation2015; Niu et al., Citation2021). The main substances in banana pseudostems that resist toppling are cellulose and lignin, with the lignin content playing a dominant role (Long et al., Citation2019). The fundamental measure to improve the ability of banana to resist tipping is to improve the mechanical bearing capacity of the pseudostems of banana varieties and to produce varieties with high resistance to typhoons. Therefore, 4CL plays an indispensable role in the process of lignin synthesis, and lignin plays an important role in the mechanical bearing capacity of banana pseudostems, so the study of 4CL is important to improve the resistance of banana pseudostems to tipping. 4CL is one of the important enzymes in the lignin synthesis process. Fourteen 4CL or similar genes have been identified in the Arabidopsis genome, of which only the proteins encoded by 4CL1, 4CL2, 4CL3 and 4CL5 are catalytically active, three are related to lignin synthesis, while only one is related to flavonoid synthesis, and the function of the remaining genes is unknown (Chowdhury et al., Citation2013); 29 4CL genes were identified in the Dangshan pear genome, and the evolutionary tree divided them into two classes, with similar genetic structures in the same class (Yunpeng et al., 2015). The Citrus aurantium genome has three 4CL genes, only Cit4CL1 is related to flavonoid biosynthesis, and Cit4CL2 and Cit4CL3 are related to lignin biosynthesis (Wanxia et al., Citation2019); 18 4CL genes were found in the genome of Populus hirsutus, and the evolutionary tree divided them into two groups with similar genetic structures in the same group (Guo-Dong & Hai, Citation2012).
Transformation of Mu4CL15 in banana seedlings revealed that both pseudostem lignin content and pseudostem physical strength were significantly higher than those of banana seedlings transformed only in null load, concluding that the 4CL gene could increase the banana’s ability to resist felling (Shenghe et al., Citation2017). Overexpression of Mu4CL15 in tobacco was found to increase the lignin content and the resistance of tobacco plants to stems (Haiyan et al., Citation2017). Overexpression of Fm4CL2 gene in Fraxinus mandshurica enhanced plant tolerance to drought and osmotic stress (Xiaohui et al., Citation2020). Overexpression of the “Dingjiaba Liguangtao” Pp4CL2 gene in Arabidopsis thaliana and tobacco enhanced plant tolerance to low temperature (Xiaolan, Citation2022). Overexpression of the 4CL binary vector from Trichoderma lucidum in tobacco revealed an increase in the number of xylem cells and the content of lignin in the stem cells of transgenic tobacco (Jiaqi et al., Citation2020). In pear, the transformation of the overexpression vectors Pb4CL11 and PbC3H1 into Arabidopsis thaliana was found to significantly increase lignin content and to promote lignin synthesis (Yunpeng et al., Citation2023).
However, most of the studies on 4CL family members have focused on individual members, and systematic analysis of the whole genome of 4CL has seldom been reported. Therefore, it is of great significance to carry out genome-wide identification and systematic analysis of the 4CL gene family. Here, we carried out a preliminary identification and categorisation of the 4CL genes in the banana A genome database, and analysed their evolutionary relationships at the genome-wide level and chromosome distribution and gene structure, laying a good foundation for the subsequent functional characterisation of banana 4CL proteins.
Methods
Data sources
Sequences of the banana 4CL protein were obtained from the banana A-genome database(Droc et al., Citation2022) (http://banana-genome/), and the 4CL gene family was retrieved by entering the keyword “coumarate” in the banana A-genome database and searching for the CDS sequences, gDNA sequences and protein sequences of the 4CL gene family members. Then, the identified 4CL gene family members of different species were further validated with the obtained banana 4CL family member sequences by BLAST on the NCBI (https://www.ncbi.nlm.nih.gov/)website to finalise the 4CL family members. The 4CL protein sequences of Arabidopsis thaliana, Oryza sativa. L, Zea mays. L and Sorghum bicolor (L.) Moench were obtained from the Phytozome database (https://phytozome.jgi.doe.gov/pz/portal.html).
The Chromosomes distribution of 4CL gene family in banana
Chromosome location mapping was conducted in Gene Location Visualize from GTF/GFF function of TBtools (Chengjie et al., Citation2020) using the Gene IDs of banana 4CL and the annotation files (gff3 files) of the Musa acuminata genomes.
The Bioinformatics analysis of 4CL protein in banana
Signal peptide prediction analysis of all members of the 4CL banana gene family was used by SignalP-5.0 (Almagro et al., Citation2019)online software (http://www.cbs.dtu.dk/services/SignalP/#opennewwindow), subcellular localisation prediction analysis was used by WoLF PSORT (Horton et al., Citation2007, software (http://psort1.hgc.jp/. form.html), NetPhos 3.1 Server (http://www.cbs.dtu.dk/services/NetPhos/) online software for phosphorylation site prediction, SOPMA(Christophe & Gilbert, Citation1995) (https://npsa-prabi.ibcp. en/cgi-bin/npsa_ automat.pl?page = npsa_sopma.html) online software for protein secondary structure prediction analysis, NetNGlyc (http://www.cbs.dtu.dk/services/NetNGlyc/) for glycosylation site prediction, and basic physicochemical properties of amino acid sequences of all members of the banana 4CL gene family by ProtParam (https://web.expasy.org/protparam/.) online software for prediction analysis.
The Structural analysis of 4CL genes in banana
The Gene Structure Display Server (http://gsds.cbi.pku.edu.cn/) online software was used to analyse the gene structure of intron and exon members of the 4CL family in combination with the CDS sequences and gDNA sequences of the banana 4CL family obtained from the banana whole genome database, and TBtools (Chengjie et al., Citation2020) was used to mapping of gene structure and conserved motifs.
Protein domains and motifs analysis search and annotation of 4CL protein in banana
The conserved motifs of the predicted banana 4CL family proteins were analysed using the online software MEME (Bailey et al., Citation2009) (http://meme-suite.org/tools/meme). The resulting conserved motifs were then functionally annotated and named using the SMART online software (http://smart.embl-heidelberg.de).
The protein–protein interaction network of the 4CL proteins in banana
The STRING (Cook et al., Citation2018) database (https://string-db.org/cgi/input.pl) was used to build the banana 4CL protein interaction network to analyse the interactions of banana 4CL proteins.
Expression Patterns of 4CL gene family under low-temperature
The FPKM values of 4CL were extracted from the low-temperature transcriptome data (Liu, Luo, et al., Citation2018) of SanMing wild plantain established in the preliminary stage of our laboratory (SRA:SRS3320042), and the expression heatmaps were plotted by using TBtools to analyse the expression of the 4CL family members at 28, 13, 4, and 0°C.
Phylogenetic Classifications of 4CL gene family in banana
The resulting 4CL proteins were subjected to multiple sequence alignment by MEGA7.0 (Kumar et al., Citation1994) software, and sequences with large homologous differences were manually deleted, and then the Neighbourhood Joining (NJ) method was used to construct phylogenetic evolutionary trees of the 4CL proteins from Musa acuminata, Arabidopsis thaliana, Oryza sativa. L, Zea mays. L and Sorghum bicolor (L.) Moench.
Collinearity analysis of 4CL gene family in banana
The banana 4CL gene family was analysed for collinearity and correlation plotted by Tbtools software to analyse gene duplication in the 4CL gene family.
Results
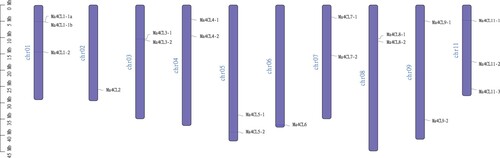
Chromosomal localisation of the banana 4CL gene family
Based on the banana genomic data, the location of the banana 4CL gene family members on the chromosomes was analysed using the Tb Tools software. As shown in , the banana 4CL gene family members were distributed one on each of chromosomes Chr2 and Chr6, two on each of chromosomes Chr1, Chr3, Chr4, Chr5, Chr7, Chr8 and Chr9, and three on chromosome Chr11. The 4CL genes were uniformly distributed and disordered in the chromosomes.
Identification and basic physicochemical characterisation of banana 4CL protein family members
Structural domains are units of protein function, each of which represents a function. When all members of a protein family contain a certain structural domain, this structural domain can be called the conserved structural domain of that protein family and constitutes the functional region of all members of the protein family with very low variability (Liu et al., Citation2020).
The prediction analysis of the conserved structural domains of the 20 protein sequences obtained by the NCBI online software classified the protein sequences of banana 4CL into five categories (https://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM = blastp&PAGE_TYPE = BlastSearch&LINK_LOC = blasthome), which showed that Ma4CL1-1a, Ma4CL1-1b, Ma4CL3-2, Ma4CL5-1, Ma4CL5-2, Ma4CL7-2, Ma4CL8-1 and Ma4CL8-2 belonged to the AFD superfamily, contained 4CL structural domain, AMP binding site and coenzyme A binding site; Ma4CL1-2, Ma4CL2, Ma4CL3-1, Ma4CL4-1, Ma4CL4-2, Ma4CL7-1, Ma4CL9-2, and Ma4CL11-3 belonged to the PLN02246 superfamily, contained 4CL structural domain, AMP binding site, and putative coenzyme A binding site; Ma4CL11-1, Ma4CL11-2 belonged to superfamily PLN02574, contained 4CL structural domain, AMP-binding site and coenzyme A-binding site; Ma4CL6 belonged to superfamily PLN02330, contained 4CL structural domain, AMP-binding site and putative coenzyme A-binding site; Ma4CL9-1 belonged to superfamily AFD, contained 4CL structural domain.
Twenty protein sequences belonged to the AFD superfamily or the PLN02246 superfamily, the PLN02574 superfamily, the PLN02330 superfamily, all contained AMP binding site, all contained 4CL structural domain, and all belonged to the banana 4CL family. The banana 4CL family contained the same evolutionarily conserved structural domain, and it can be predicted that members of the banana 4CL gene family may have the same function.
Analysis of the physicochemical properties of the banana 4CL family proteins using ExPASy online software showed that the amino acid lengths of the banana 4CL gene family members ranged from 160 to 700, with the shortest, Ma4CL9-1, having an amino acid length of only 182, and the longest, Ma4CL3-1, having an amino acid length of 655. Isoelectric points ranged from 5.20 to 9.02, with 12 acidic proteins having an isoelectric point below 7 and 8 basic proteins having an isoelectric point above 7. The isoelectric point of Ma4CL1-2 was smallest at 5.20, and Ma4CL8-2 was largest at 9.02. The instability index analysis showed that most of the 4CL family members were stable proteins, Ma4CL1-1a, Ma4CL1-1b, Ma4CL3-1, Ma4CL3-2, Ma4CL5-2, Ma4CL7-2, Ma4CL8-1, Ma4CL8-2, Ma4CL8-2 and Ma4CL8-2. 1, Ma4CL8-2, all eight proteins had an instability index greater than 40 and were unstable proteins. Analysis of the total average hydrophobicity index showed that the 4CL family members all had a positive total average hydrophobicity index, and were therefore all hydrophobic proteins ().
Table 1. Basic information about the members of the banana 4CL protein family.
The Bioinformatics analysis of 4CL protein in banana
Secondary structure analysis of the banana 4CL protein () showed that the secondary structure of 4CL was composed of four forms: α-helix, extended chain structure, β-turn and irregular loop, among which all proteins, except Ma4CL9-1, had the highest percentage of irregular loop, the lowest percentage of β-turn, and the second and third percentages of α-helix and extended chain structure, respectively. The percentages of secondary structures of Ma4CL9-1 proteins were α-helix > irregular loop > extended chain structure > β-turn. Subcellular localisation of 4CL protein family members by WoLF PSORT online software showed that most members were localised in the cytoplasmic membrane, while a few were localised in microsomes, cytoplasm, mitochondrial inner membrane, nucleus and endoplasmic reticulum. The highest probability of localisation in the cytoplasmic membrane was 0.79 for Ma4CL1-1a, Ma4CL1-1b, Ma4CL3-1, Ma4CL5-1, Ma4CL6 and Ma4CL7-1; the highest probability of localisation in microsomes was 0.82 for Ma4CL1-1a; the highest probability of localisation in the endoplasmic reticulum was 0.82 for Ma4CL1-2, Ma4CL4-2 and Ma4CL4-2. Ma4CL4-2; Ma4CL11-2 in the mitochondrial inner membrane; Ma4CL7-2 and Ma4CL9-2 in the cytoplasm; and Ma4CL4-1 in the nucleus.
Signal peptide predictions for the banana 4CL protein family were performed using the online software SignalP-5.0, which showed no predicted signal peptide sequences for all members.
The serine, threonine and tyrosine phosphorylation sites of the 4CL protein family members were analysed and predicted by the online software NetPhos 3.1, and the results are presented in . The phosphorylation sites of the banana 4CL protein family members ranged from 10 to 60, with Ma4CL8-2 having the highest number of phosphorylation sites (56) and Ma4CL9-1 having the lowest (14). The number of phosphorylation sites for all family members was Ser (serine) > Thr (threonine) > Tyr (tyrosine), showed that the phosphorylation sites for all members of the banana 4CL protein family were predominantly Ser (serine). Ma4CL8-1 has the highest serine (S) number at 37 and Ma4CL9-1 has the lowest serine (S) number at 6. Ma4CL6 has the highest threonine (T) number at 21 and Ma4CL9-1 has the lowest threonine (T) number at 4. All members of the banana 4CL protein family contained serine, threonine and tyrosine sites, but the number of these three types of sites in each protein varies. It is assumed that the expression of function and activity varies between the 20 members of the 4CL protein family.
The N-glycosylation sites and O-glycosylation sites of the 4CL protein family were analysed and predicted by the online software NetNGlyc and NetOGlyc, and can be obtained. From the data analysed in , it can be concluded that the banana 4CL protein members had significantly more O-glycosylation sites than N-glycosylation sites. The two members, Ma4CL1-1a and Ma4CL1-1b, had no N-glycosylation sites. Ma4CL8-2 was the member of the 4CL protein family with the most N-glycosylation sites, with six N-glycosylation sites. In contrast, the maximum number of O-glycosylation sites was much higher than the number of N-glycosylation sites, at 12. Only Ma4CL7-2 had no potential O-glycosylation sites, while nine members of the protein had no potential N-glycosylation sites.
Table 2. Prediction of 4CL glycosylation sites in banana.
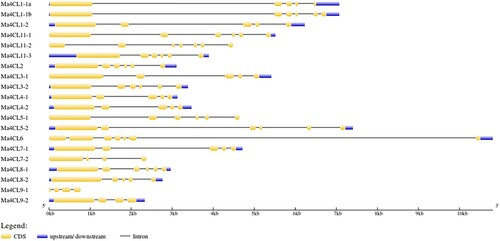
The Structural analysis of 4CL gene family in banana
Analysis of the 4CL gene structure used GSDS online software showed that all other members of the 4CL gene family were composed of a UTR region, introns and exons, except for four members, Ma4CL5-1, Ma4CL7-2, Ma4CL9-1 and Ma4CL11-2, which do not contain a UTR region. Three members, Ma4CL7-2, Ma4CL9-1 and Ma4CL9-2, contained three introns and four exons, seven members, Ma4CL1-1a, Ma4CL1-2, Ma4CL3-1, Ma4CL4-1, Ma4CL4-2, Ma4CL7-1 and Ma4CL8-2, contained four introns and five exons. The eight members Ma4CL1-1b, Ma4CL2, Ma4CL3-2, Ma4CL5-1, Ma4CL5-2, Ma4CL8-1, Ma4CL11-1, Ma4CL11-3 contained 5 introns and 6 exons, and the two members Ma4CL6 and Ma4CL11-2 contained 6 introns and 7 exons ().
The Protein domains and motifs analysis of 4CL Proteins in banana
The conserved motifs of banana 4CL family proteins were predicted using the online analysis software MEME, setting the maximum motif to 20 and the length to 6∼300 amino acids, and the images were plotted using the Tb tool, as shown in . The results show that all members of the banana 4CL protein family contain motif 1, except for Ma4CL1-1b, Ma4CL9-1 and Ma4CL11-2; And the eight proteins Ma4CL1-2, Ma4CL2, Ma4CL3-1, Ma4CL4-1, Ma4CL4-2, Ma4CL7-1, Ma4CL9-2 and Ma4CL11-3 all contained motif 2, all belonged to the same family and may have the same function.
Figure 3. Conserved motifs distribution of banana 4CL family members. The different colors represent the different motifs.
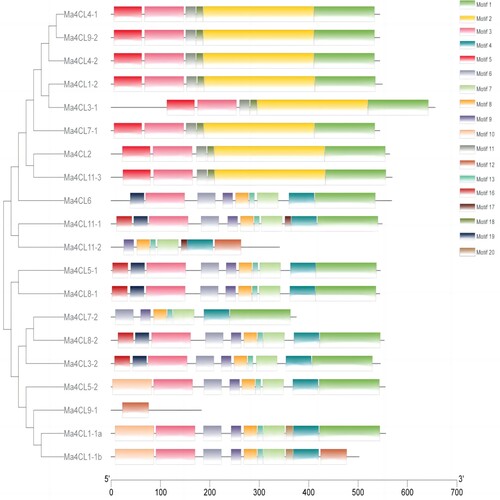
Five of the predicted conserved motifs were named and functionally annotated by the SMART online software, and the results are presented in . In combination with the structure of the 4CL protein, it was concluded that motif 1 belonged to the SAP (DNA binding) domain and was mainly involved in DNA binding associated with chromosome organisation (double helix); Eight of the protein members contained motif 2 and belonged to the ZU5 domain, the Molybdop_Fe4S4 domain. And, the Molybdop_Fe4S4 domain (molybdopterin oxidoreductase 4Fe-4S structural domain) was present in many reductase/dehydrogenase families. Hu et al. analysed the poplar 4CL gene family and concluded that 4CL genes containing both the two conserved structural domains of SSGTTGLPKGV (AMP-binding region) and GEICIRG (catalytic site of the enzyme) were required to regulate lignin synthesis (Hu et al., Citation1998; Wanxia et al., Citation2019), while 4CL genes that do not contain both the two conserved structural domains of SSGTTGLPKGV and GEICIRG were involved in flavonoid synthesis (Cukovica et al., Citation2001). The 4CL genes involved in lignin synthesis that have been reported so far both contain conserved structural domains SSGTTGLPKGV and GEICIRG (Luo et al., Citation2016). In this study, eight members of the banana 4CL gene family containing motif 2 contained both SSGTTGLPKGV and GEICIRG conserved structural domains, which all belonged to class I. Therefore, they were assumed to be involved in lignin synthesis.
Table 3. Sequence and functional annotation of conserved motifs of banana 4CL.
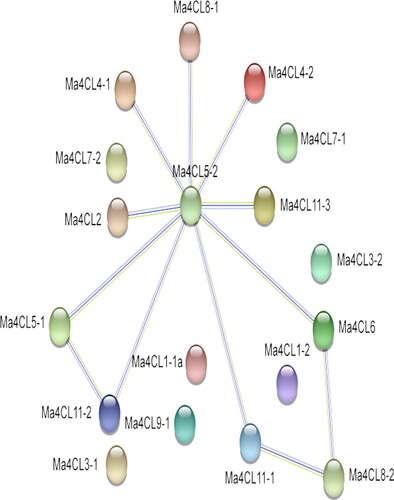
The protein–protein interaction network of the 4CL proteins in banana
The protein interactions of the banana 4CL family members were predicted by the STRNG online software () and the results showed that the banana 4CL protein family members were partially interoperable, with Ma4CL5-2 being the main central node of the protein network, with which most other 4CL family members were either strongly or weakly associated, and that the Ma4CL5-2 protein functions in relation to the AMP-binding domain, which was a Ser/Thr/Gly-rich structural domain that was also characterised by the conserved Pro-Lys-Gly triplet. This family of enzymes included luciferase, long chain fatty acid Co-A ligases, acetyl coenzyme A synthase and various other closely related synthases. Other proteins function primarily in flavonoid biosynthesis, ubiquinone and other terpenoid quinone biosynthesis, phenylpropane biosynthesis and the phenylalanine metabolic pathway.
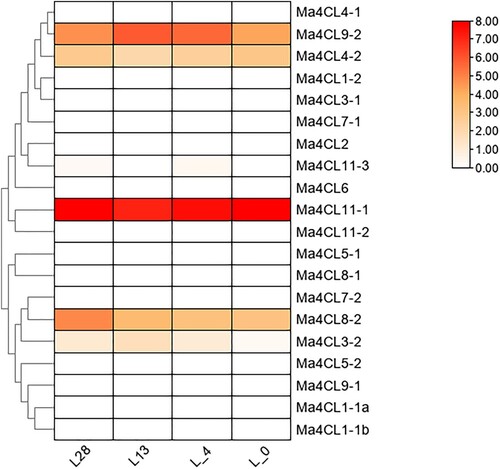
Expression Patterns of 4CL gene family under low-temperature
The transcriptome FPKM data were used to analyse the expression patterns of 4CL, the 4CL genes were analysed and visualised using the Tbtools, as shown in . The expression of Ma4CL3-2 was up-regulated at a low temperature of 13°C, down-regulated at a low temperature of 4°C and down-regulated at a low temperature of 0°C; Ma4CL4-2 expression was down-regulated at a low temperature of 13°C, up-regulated at a low temperature of 4°C and up-regulated at a low temperature of 0°C; Ma4CL8-2 expression was down-regulated at a low temperature of 13°C, up-regulated at a low temperature of 4°C and up-regulated at a temperature of 0°C. low temperature; Ma4CL8-2 was down-regulated at 13°C low temperature and its expression was largely unchanged at 4°C and 0°C low temperature; Ma4CL9-2 was up-regulated at 13°C low temperature, its expression was largely unchanged at 4°C low temperature and its expression was down-regulated at 0°C low temperature; Ma4CL11-1 was down-regulated at 13°C low temperature, its expression was up-regulated at 4°C low temperature and its expression was up-regulated at 0°C low temperature. The expression of Ma4CL11-1 was down-regulated at 13°C, up-regulated at 4°C and up-regulated at 0°C. The expression of Ma4CL3-2 was relatively low, followed by Ma4CL4-2, the expression of Ma4CL8-2 and Ma4CL9-2 was relatively high, and the expression of Ma4CL11-1 was relatively the highest. In addition, the other members of Ma4CL were barely expressed.
The Phylogenetic Classifications of 4CL gene family in banana
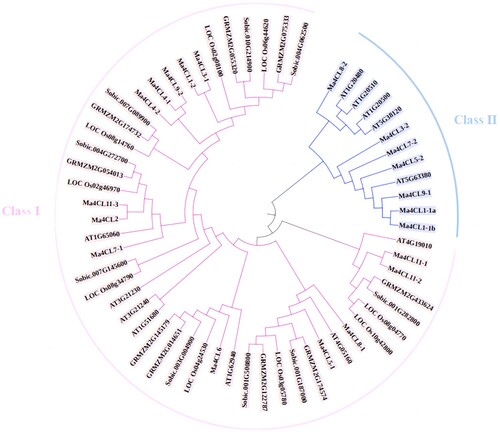
A phylogenetic tree was constructed by comparing the 4CL protein family of banana (Musa acuminata) with the 4CL protein sequences of Arabidopsis thaliana, Oryza sativa. L, Zea mays. L and Sorghum bicolor (L.) Moench. The results are shown in . These 59 4CL proteins can be divided into 2 subfamilies, of which Class I contained 13 banana 4CL members and Class II contained 7 banana 4CL members. Arabidopsis thaliana was distributed in 2 subfamilies as reported, while Oryza sativa. L, Zea mays. L and Sorghum bicolor (L.) Moench. were only distributed in Class I, and only banana (Musa acuminata) and Arabidopsis thaliana 4CL proteins were in Class II. The reason for this may be related to the process of monodicotyledonous differentiation in plants. Combined with the above analysis, it can be concluded that the eight members containing motif 2 are all distributed in Class I and may have the same function.
Figure 6. The phylogenetic analysis of 4CL gene family between Musa acuminata, Arabidopsis thaliana, Oryza sativa. L, Zea mays. L and Sorghum bicolor (L.) Moench. The phylogenetic tree was constructed by MEGA7.0 with the Neighbourhood Joining (NJ) method. Different clades were painted with different colors.
The Collinearity analysis of 4CL gene family in banana
Based on the banana genome data, the duplication of 4CL gene family members in the Musa acuminata、Musa balbisiana、Musa itinerans genomes was analysed using the Tb tools software. As shown in and , the colinear gene pairs were evenly distributed, with 22 colinear pairs between the Musa acuminata and Musa balbisiana genomes and 19 colinear pairs between the Musa acuminata and Musa itinerans genomes. There were slightly more colinear gene pairs between the Musa acuminata and Musa balbisiana genomes than between the Musa acuminata and Musa itinerans genomes, indicated that the evolutionary relationship between the Musa acuminata and Musa balbisiana genomes was closer than that between the Musa acuminata and Musa itinerans genomes.
Figure 7. Interspecies collinear analysis of 4CL genes in the whole genome of Musa acuminata, Musa balbisiana and Musa itinerans. The green represents the Musa balbisiana, the orange represents the Musa acuminata, and the blue represents the Musa itinerans. The red curve between chromosomes represents the gene pairs between banana 4CLs.
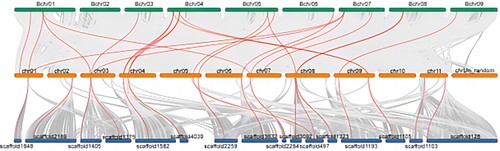
Table 4. Colinear pairs of the 4CL gene family in banana.
Discussion
Members of the banana 4CL gene family may be closely linked to the process of plant differentiation into mono-dicotyledons
In this study, a total of 20 members of the banana 4CL gene family were identified by genome-wide identification of the 4CL gene family in the Musa acuminata genome. Prediction analysis of the conserved structural domains of these members showed that they all contained 4CL structural domains, all belonged to the 4CL family and all contained AMP binding sites. The banana 4CL gene family contains the same evolutionarily conserved structural domains, and it is assumed that the banana 4CL gene family members have the same functions. In combination with the analysis of the Arabidopsis thaliana 4CL gene family, the functions of the banana 4CL gene family were assumed to be related to lignin synthesis. Conserved motif prediction analysis revealed that motif 1 of the banana 4CL protein family belonged to the SAP domain, which was mainly involved in DNA binding function; eight members of the protein contained motif 2, all of which belonged to class I and had the same function. Phylogenetic tree analysis combined Arabidopsis thaliana, Zea mays, Oryza sativa, Sorghum bicolor (L.) Moenchand 4CL protein sequences are classified into two main classes, class I containing 13 banana 4CL protein sequences and class II containing 7. Huang Shengxiong et al. (Huang et al., Citation2008) classified plant 4CL genes into two main classes: the 4CL genes of dicots, and the 4CL genes of monocots and gymnosperms. The results of this study are similar to those of previous studies, and the fact that class II contains only banana and Arabidopsis thaliana protein sequences may be due to the fact that 4CL genes are closely related to the evolutionary process of plants, and that the presence of 4CL genes in plants may be related to the differentiation process of monocots and dicots.
In this study, preliminary predictive analyses of the banana 4CL gene family in terms of physicochemical properties, secondary structure, signal peptide, protein structure, phosphorylation sites, glycosylation sites and conserved motifs used bioinformatics methods revealed that the members of the gene family were relatively diverse in terms of structure, and it was hypothesised that they may be related to plant lignin synthesis and be closely associated with the process of plant differentiation into mono-dicotyledons. These results provided a basis for further studies on the regulation of the expression, structure and function of this gene family.
Eight members of the banana 4CL gene family Class I are likely to be involved in lignin synthesis, while the rest are likely to be involved in flavonoid synthesis
Lignin synthesis is an evolutionary expression of the adaptation of land plants to their environment and plays an irreplaceable role in plant growth and development. The synthesis of lignin aggregates in plant cells plays an important role in the mechanical strength of the plant, the maintenance of normal cell morphology, the evacuation of water and resistance to foreign pathogens. The mechanical strength of banana pseudostems has a great impact on banana production, and a strong banana pseudostem has a much higher fruit production capacity. Bananas grown in China are prone to damage to banana leaves in summer due to typhoons, which can cause severe pseudostem collapse, leading to massive yield reductions in the banana industry. While lignin is one of the most important macromolecules in wood besides cellulose, which is inherently soft, lignin is a key factor in improving the mechanical strength of banana pseudostems (Hussain et al., Citation2018) and the lodging resistance of banana. And lignin synthesis is closely linked to the 4CL gene. In recent years, more and more 4CL gene sequences from plants have been cloned one after another(Xiao-Ming et al., Citation2017), but little research has been done on the banana 4CL gene family. In this study, eight banana 4CL gene family member proteins containing motif 2 were found to contain both SSGTTGLPKGV and GEICIRG conserved structural domains belonged to the PLN02246 superfamily, all of which belonged to class I. Therefore, it was assumed that they could be involved in lignin synthesis. In contrast, the other five members of the banana 4CL protein of class I and all members of the banana 4CL protein of class II could be involved in flavonoid synthesis.
Conclusion
In this study, it is assumed that some members of the banana 4CL gene family may play an important role in banana resistance to lodging. Cloning, expression analysis and functional validation of relevant genes can be carried out at a later stage to lay the foundation for banana breeding.
Declaration of competing interests
The authors declare no conflict of competing interests.
Acknowledgements
All authors contributed to the study’s conception and design. ZhongXiong Lai presented the thesis topics and gave valuable advice, MengGe Wang analysed the data, created the figures and wrote the paper, Liang Jia to make additions and revisions to the content of the paper, YanYing Liu and ChunYu Zhang gave useful advice and provided the language assistance. ZhongXiong Lai, YuJi Huang, YuKun Chen and YuLing Lin revised the paper. All authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Almagro, A. J. J., Tsirigos, K. D., Sønderby, C. K., Petersen, T. N., Winther, O., Brunak, S., von Heijne, G., & Nielsen, H. (2019). Signalp 5.0 improves signal peptide predictions using deep neural networks. Nature Biotechnology, 37(4), 420–423. https://doi.org/10.1038/s41587-019-0036-z
- Bailey, T. L., Boden, M., Buske, F. A., Frith, M., Grant, C. E., Clementi, L., Ren, J., Li, W. W., & Noble, W. S. (2009). MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Research, 37(Web Server issue), W202–W208. https://doi.org/10.1093/nar/gkp091
- Behr, M., Guerriero, G., Grima-Pettenati, J., & Baucher, M. (2019). A molecular blueprint of lignin repression. Trends in Plant Science, 24(11), 1052–1064. https://doi.org/10.1016/j.tplants.2019.07.006
- Cao, Y. P., Fang, Z., Li, S. M., Cc, Y., Qq, D., Cheng, X., Lin, Y., Guo, N., & Yp, C. (2015). Genome-wide identification and analyses of 4CL gene families in Pyrus bretschneideri. Hereditas, 37(7), 711–719. https://doi.org/10.16288/j.yczz.15-069
- Chen, Y. L. (2009). Cloning and RNAi vector construction of gene fragments related to lignin metabolism of Miscanthus. [D].Fuzhou: Fujian Agriculture and Forestry University. https://kns.cnki.net/kcms/detail/detail.aspx?FileName=2009170507.nh&DbName=CMFD2009.
- Chengjie, C., Hao, C., Yi, Z., Hannah, R. T., Margaret, H. F., Yehua, H., & Rui, X. (2020). Tbtools: An integrative toolkit developed for interactive analyses of Big biological data. Molecular Plant, 2020, 13(8), 1194–1202. https://doi.org/10.1016/j.molp.2020.06.009
- Chowdhury, M. E. K., Choi, B., Cho, B. K., Kim, J. B., Park, S. U., Natarajan, S., Lim, H., & Bae, H. (2013). Regulation of 4CL, encoding 4-coumarate:coenzyme A ligase, expression in kenaf under diverse stress conditions. Plant Omics, 6, 254–262. https://api.semanticscholar.org/CorpusID:15581879
- Christophe, G., & Gilbert, D. (1995). SOPMA: Significant improvements in protein secondary structure prediction by consensus prediction from multiple alignments. Computer Applications in the Biosciences, 11(6), 681–684. https://doi.org/10.1093/bioinformatics/11.6.681
- Cook, H. V., Doncheva, N. T., Szklarczyk, D., von Mering, C., & Jensen, L. J. (2018). Viruses.STRING: A virus-host protein-protein interaction Database. Viruses-Basel, 10(10), 519. https://doi.org/10.3390/v10100519
- Cukovica, D., Ehlting, J., Ziffle, J. A. V., & Douglas, C. J. (2001). Structure and evolution of 4-coumarate:coenzyme a ligase (4CL) Gene Families. Biological chemistry, 382(4), 645–654. https://doi.org/10.1515/BC.2001.076
- Droc, G., Martin, G., Guignon, V., Summo, M., Sempéré, G., Durant, E., Soriano, A., Baurens, F., Cenci, A., Breton, C., Shah, T., Aury, J., Ge, X., Harrison, P. H., Yahiaoui, N., D'Hont, A., & Rouard, M. (2022). The banana genome hub: A community database for genomics in the Musaceae. Horticulture Research, 9 uhac221, https://doi.org/10.1093/hr/uhac221
- Fan, Rui., Hu, Lisong., Wu, Baoduo., HAO,Chaoyun. (2020). Cloning and expression analysis of 4-coumarate:coenzyme A ligase gene (Pn4CL) in piper nigrum [J]. Chinese Journal of Tropical Crops, 41(4), 737–744. https://doi.org/10.3969/j.issn.1000-2561.2020.04.015
- Gaige, Z., Jianhui, L., Erpei, L., & Yongquan, L. (2020). Cloning of Cl4CL gene and lts expression analysis in Chinese Fir. Molecular Plant Breeding, 18(6), 1832–1837. https://doi.org/10.13271/j.mpb.018.001832
- Gao, H., Guo, D. M., Liu, W. J., Ran, J. H., & Wang, X. Q. (2012). Evolution of the 4-coumarate:coenzyme A ligase (4CL) gene family: Conserved evolutionary pattern and two new gene classes in gymnosperms. Journal of Systematics and Evolution, 50(3), 195-205. https://doi.org/10.1111/j.1759-6831
- Gao, Z. N. (2015). Lodging resistance and the response to cultivation measures of oilseed flax[D]. Lanzhou: Gansu Agricultural University. https://kns.cnki.net/kcms/detail/detail.aspx?FileName=1015975610.nh&DbName=CDFD2016.
- Guo, L. L. (2015). Cloning and analysis of 4CL genes in tea plant [Camellia sinensis (L.)][D]. Hefei: Anhui Agricultural University. https://kns.cnki.net/kcms/detail/detail.aspx?FileName=1016064221.nh&DbName=CMFD2017.
- Guo-Dong, R., & Hai, L. U. (2012). Cloning and phylogenetic analysis of 4cl gene family from populus tomentosa carr. Guangdong Agricultural Sciences, (8), 141–144. https://doi.org/10.16768/j.issn.1004-874x.2012.08.064
- Haiyan, S., Wei, S., Qing, W., Zhan, W., Manni, Y., Qian, H., Zhiqiang J., JingyangL., Shenghe C. (2017). Functional identification of mu4cl15 in 4cl gene in banana. Genomics and Applied Biology, (8), 3025–3033. https://doi.org/10.13417/j.gab.036.003025
- Horton, P., Park, K., Obayashi, T., Fujita, N., Harada, H., Adams-Collier, C. J., & Nakai, K. (2007). WoLF PSORT: Protein localization predictor. Nucleic Acids Research, 35(Web Server issue), W585–W587. https://doi.org/10.1093/nar/gkm259
- Hu, W. J., Kawaoka, A., Tsai, C. J., Lung, J., Osakabe, K., Ebinuma, H., & Chiang, V. L. (1998). Compartmentalized expression of two structurally and functionally distinct 4-coumarate:CoA ligase genes in aspen (Populus tremuloides). Proceedings of the National Academy of Sciences of the United States of America, 95(9), 5407–5412. https://doi.org/10.1073/pnas.95.9.5407
- Huang, S., Hu, S., Sun, X., Cao, Y., Lu, X., & Jiang, Y. (2008). Genetic and evolutionary analysis of lignin biosynthase 4CL gene. Journal of Northwest AandF University (Natural Science Edition, (10), 199–206. https://doi.org/10.13207/j.cnki.jnwafu.2008.10.005
- Hussain, M., Farooq, S., Hasan, W., Ul-Allah, S., Tanveer, M., Farooq, M., & Nawaz, A. (2018). Drought stress in sunflower: Physiological effects and its management through breeding and agronomic alternatives. Agricultural Water Management, 201, 152–166. https://doi.org/10.1016/j.agwat.2018.01.028
- Jiaqi, H., Qi, Q., Xiangning, J., & Ying, G. (2020). Effect of Fusion Gene 4CL1-CCR of Populus tomentosa on Lignin Deposition in Tobacco. Scientia Silvae Sinicae, 56(10), 63–69. https://doi.org/10.11707/j.1001-7488.20201007
- Jing, L., Feng, X. U., Xiao-Hui, W., Wei-Wei, Z., & Yong-Ling, L. (2016). Molecular cloning and expression analysis of 4-coumarate:CoA ligase gene in ginkgo biloba. Journal of Yangtze University(Natural Science Edition), (15), 42-48+5-6. https://doi.org/10.16772/j.cnki.1673-1409.2016.15.010
- Jinshan, G., Junhui, S., & Laigeng, L. (2011). Functional characterization of evolutionarily divergent 4-coumarate:coenzyme a ligases in rice. Plant Physiology, 157(2), 574–586. https://doi.org/10.1104/pp.111.178301
- Jullyana, C. M. E. S., Cesar, A. V. B., Juliana, D. O. F. V., Marcelo, C. D., & Paulo, M. (2010). Abiotic and biotic stresses and changes in the lignin content and composition in plants. Journal of Integrative Plant Biology, 52(4), 360–376. https://doi.org/10.1111/j.1744-7909.2010.00892.x
- Kumar, S., Tamura, K., & Nei, M. (1994). Mega: Molecular evolutionary genetics analysis software for microcomputers. Computer Applications in the Biosciences: Cabios, 10(2). 189–191. https://doi.org/10.1093/bioinformatics/10.2.189
- Lavhale, S. G., Kalunke, R. M., & Giri, A. P. (2018). Structural, functional and evolutionary diversity of 4-coumarate-CoA ligase in plants. Planta, 248(5), 1063–1078. https://doi.org/10.1007/s00425-018-2965-z
- Li, Y., Yuan, X., Wang, Y., & Zhang, J. (2021). Cloning and expression analysis of 4CL gene from betula alnoides. Molecular Plant Breeding, 19(1), 118–122. https://doi.org/10.13271/j.mpb.019.000118
- Li, Z. (2009). Genetie manipulation of Lignin Biosynthesis in Poplar [ M.S., Capital Normal University]. https://kns.cnki.net/kcms/detail/detail.aspx?FileName=2009129895.nh&DbName=CMFD2009.
- Liu, Q., Luo, L., & Zheng, L. (2018). Lignins: Biosynthesis and biological functions in plants. International Journal of Molecular Sciences, 19(2), 335-335. https://doi.org/10.3390/ijms19020335
- Liu, W., Cheng, C., Lin, Y., XuHan, X., & Lai, Z. (2018). Genome-wide identification and characterization of mRNAs and lncRNAs involved in cold stress in the wild banana (Musa itinerans). PLoS One, 13(7), e0200002. https://doi.org/10.1371/journal.pone.0200002
- Liu, X., Zeng, Y., Li, D., & Zheng, Y. (2020). Bioinformatics analysis of plant hormone-related genes in oil palm. Molecular Plant Breeding, 18(11), 3493–3501. https://doi.org/10.13271/j.mpb.018.003493.
- Liu, X., Zhang, L., Xing, J., & Liang, Y. (2013). Cloning and evolutionary analysis of homologous sequences of 4 CL gene in pinus massoniana. Guizhou Agricultural Sciences, 41(12), 28–31. https://doi.org/10.3969/j.issn.1001-3601.2013.12.008
- Long, F., Wu, P., Zhao, M., He, H., & Zou, Y. (2019). Lodging resistance of different genotypes of cultivated bananas based on physical and chemical properties of pseudo stem. Southwest China Journal of Agricultural Sciences, 32(9), 2157–2162. https://doi.org/10.16213/j.cnki.scjas.2019.9.029
- Luo, R., Zhao, Z., Gao, A., Huang, J., & Liu, K. (2016). Cloning and expression analysis of 4CL gene from mango (Mangifera indica). North China Journal of Agriculture, 31(S1), 57–62.
- Meng, L., Tasiken, H., Zhuo, C., Zhili, T., & Chao, W. (2017). Cloning and Expression Analysis of 4CL Gene Promoter of Betula platyphylla. Chinese Agricultural Science Bulletin. 2017, 33(5), 29–34. https://doi.org/10.11924/j.issn.1000-6850.casb16040031
- Moti, A., Miriam, E., & Hagai, A. (2012). Removing allosteric feedback inhibition of tomato 4-coumarate:CoA ligase by directed evolution. The Plant Journal: For Cell and Molecular Biology, 69(1), 57–69. https://doi.org/10.1111/j.1365-313X.2011.04770.x
- Niu, Y., Chen, T., Zhao, C., & Zhou, M. (2021). Improving crop lodging resistance by adjusting plant height and stem strength. Agronomy, 11(12), 2421. https://doi.org/10.3390/agronomy11122421
- Shenghe, C., Wei, S., Guiying, X., Qing, W., Jingyang, L., Haiyan, S. (2017). Enhancing lodging resistance of banana plant by transforming 4-coumarate:CoA ligase gene mu4cl15. Molecular Plant Breeding, (12), 4905–4911. https://doi.org/10.13271/j.mpb.015.004905
- Shengxiong, H., Shanglian, H., Xia, S., Ying, C., Xueqin, L., & Yao, J. (2008). Genetic and evolutionary analysis of lignin biosynthase 4CL gene. Journal of Northwest AandF University (Natural Science Edition, (10), 199–206. doi:10.13207/j.cnki.jnwafu.2008.10.005
- Sun, S., & Sun, J. (2020). Characterization of the Gh4CL gene family reveals a role of Gh4CL7 in drought tolerance. 19th annual conference of the crop society of China, Wuhan, Hubei, People’s Republic of China. https://kns.cnki.net/kcms/detail/detail.aspx?FileName=CSSC202011001153&DbName=CPFD2021.https://doi.org/10.26914/c.cnkihy.2020.047882
- Wang, X., Sun, X., Chi, J., Shi, X., & Cheng, C. (2020). Cloning and expression analysis of two 4CL genes in gerbera. Chinese Journal of Applied and Environmental Biology, 26(2), 272–279. https://doi.org/10.19675/j.cnki.1006-687x.2019.07025
- Wanxia, S., Zhibin, W., Yang, X., Wantong, Z., Shiping, Z., & Xiaochun, A. Z. (2019). Characterization of 4-coumarate:CoA ligase(4cl)gene family in citrus. Acta Horticulturae Sinica, (6), 1068–1078. https://doi.org/10.16420/j.issn.0513-353x.2018-0993
- Xiang, P., Huanhuan, L., Hongyi, W., Wankai, S., Xiangning, J., & Hai, L. (2013). Analysis of the spatial and temporal expression pattern directed by the Populus tomentosa 4-coumarate:CoA ligase Pto4CL2 promoter in transgenic tobacco. Molecular Biology Reports, 40(3), 2309–2317. https://doi.org/10.1007/s11033-012-2312-6
- Xiaohui, C., Wenlong, S., Han, Z., Yaguang, Z., & Fansuo, Z. (2020). Fraxinus mandshurica 4-coumarate-CoA ligase 2 enhances drought and osmotic stress tolerance of tobacco by increasing coniferyl alcohol content. Plant physiology and biochemistry : PPB, 155, 697–708. https://doi.org/10.1016/j.plaphy.2020.08.031
- Xiaolan, L. (2022). Response of Dingjiaba to low-temperature stress and Pp4CL2 function verification [M.S., Gansu Agricultural University]. https://link.cnki.net/doi/10.27025/d.cnki.ggsnu.2022.000315.
- Xiao-Ming, T., Li-Hong, Y., Guang-Feng, X., & Li-Yuan, J. (2017). Research progress on 4-coumarate:coenzyme A ligase(4CL) in plants. Biotechnology Bulletin, (4), 19–26. https://doi.org/10.16420/j.issn.0513-353x.2018-0993
- Xinting, L., Juan, W., Xianfei, H., Qiang, L., Donghai, J., Yuanguo, G., & Haiyan, L. (2019). Progress of functions of lignin and relevant genes in plant lodging resistance. Molecular Plant Breeding, (2), 655–662. https://doi.org/10.13271/j.mpb.017.000655
- Yunpeng, C., Yu, C., Lin, Z., & Yongping, C. (2023). Two monolignoid biosynthetic genes 4-coumarate:coenzyme A ligase (4CL) and p-coumaric acid 3-hdroxylase (C3H) involved in lignin accumulation in pear fruits. Physiology and Molecular Biology of Plants, 29(6), 791–798. https://doi.org/10.1007/s12298-023-01329-1
- Zhao, Q. (2016). Lignification: Flexibility, biosynthesis and regulation. Trends in plant science, 21(8), 713–721. https://doi.org/10.1016/j.tplants.2016.04.006
- Zhao, Q., & Dixon, R. (2011). Transcriptional networks for lignin biosynthesis: more complex than we thought?. Trends in plant science, 16(4), 227–233. https://doi.org/10.1016/j.tplants.2010.12.005