?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The occurrence of sequelae is common after heatstroke. There is a lack of risk prediction data regarding the prevalence of sequelae. This study aims to develop a predictive model for the sequelae after heatstroke, including cerebellar dysfunction. Heatstroke patients admitted to our hospital in the past 8 years were retrospectively enrolled. Regression analyses were conducted to identify risk factors associated with sequelae and cerebellar dysfunction. A prediction model for post-heatstroke sequelae was developed and internally and externally validated. Twenty-three percent of the patients experienced sequelae, with cerebellar dysfunction accounting for 51.7%. Patients with sequelae had longer duration of hyperthermia and coma, lower Glasgow Coma Scales scores, elevated core temperature, increased incidence of multiple organ dysfunction upon admission, and a worse prognosis compared to non-sequelae patients. Patients with cerebellar injury exhibited impaired balance, and a Sepsis-related Organ Failure Assessment (SOFA) score > 5 is an independent risk factor. The prediction system included advanced age, coma, and an APACHE-II score > 10 upon admission, predicting sensitivity = 0.8966, specificity = 0.8925, and AUC of ROC curve = 0.941 (95% CI: 0.884 ~ 0.976). The C-index in the internal verification was 0.942 (95% CI: 0.847 ~ 0.981) and the AUC in the external verification was 0.816 (95% CI: 0.699 ~ 0.902). The prediction system included advanced age, coma, and higher APACHE-II scores as variables, indicating a significant prevalence of sequelae. Higher SOFA scores were associated with the increased occurrence of cerebellar dysfunction and balance impairment. The prediction system could better predict sequelae after heatstroke.
Introduction
Heatstroke (HS) is diagnosed by the presence of hyperthermia and neurological impairment. The primary clinical manifestation of HS is central nervous system (CNS) dysfunction, characterized by prolonged coma, quadriparesis, ataxia, and cognitive impairment. The occurrence of neurological sequelae, particularly cerebellar injury, was frequently observed in survivors of HS and proved challenging to prevent even with timely treatment (Moshe et al., Citation2007). About 23.3% of the HS patients suffered from convalescent or long-term neurological sequelae and 71.4% of them had long-term cerebellar dysfunction (Lawton et al., Citation2019). However, the early period of HS often presents few detectable risk factors for the development of sequelae, leading to delayed diagnosis and treatment and ultimately resulting in an unfavorable prognosis.
The occurrence of sequelae after HS involves various types, with 34.4% of survivors experiencing permanent neurological deficits. Among these impairments, the predominant neurological impairment observed is cerebellar ataxia (Lawton et al., Citation2019). Pancerebellar syndrome after HS is irreversible and is shown up as generalized cerebellar atrophy in magnetic resonance imaging (MRI) (Yaqub & Al Deeb, Citation1998). The identification and initiation of treatment for cerebellar damage typically occur 1 month later after HS (Bazille et al., Citation2005). Cerebellar atrophy could be shown for 10 weeks after HS is accompanied by persistent ataxia that resulted in the patient being confined to a wheelchair (Albukrek et al., Citation1997). The presence of brain injury due to HS may indicate an unfavorable prognosis (Kosgallana et al., Citation2013). However, the lack of sufficient indicators for early prediction of cerebellar injury leads to a delay in the initiation of treatment. In addition to neurological sequelae, heatstroke patients exhibited a 1.8-fold increased risk of long-term cardiovascular diseases compared to non-heatstroke patients, including ischemic heart disease, heart failure, and atrial fibrillation (Nzvere et al., Citation2020). The formation of atherosclerosis and heart failure is associated with delayed metabolic disturbances occurring in exertional HS mice (Nzvere et al., Citation2020). Patients with HS may also experience subsequent mental health conditions, infertility, chronic fatigue, and other related symptoms in the later stages of the HS. Therefore, the identification of early predictive risk factors and prompt initiation of treatment is crucial in mitigating long-term sequelae following HS.
HS can lead to multiple organ dysfunction syndrome (MODS), including disorder of consciousness, acute respiratory distress syndrome, hepatic and renal dysfunction, disseminated intravascular coagulation (DIC), rhabdomyolysis, and even death (Leon & Bouchama, Citation2015). The patient may exhibit edema, congestion, and hemorrhagic necrosis in the brain, lungs, heart, liver, spleen, kidneys, and gut as pathological manifestations of HS (Mahri & Bouchama, Citation2018). The relationship between MODS caused by HS and the subsequent development of multiple organ sequelae remains unclear. Therefore, the objective of this study was to identify the risk factors associated with sequelae during the early stage of HS and develop a predictive model for sequelae based on MODS.
Methods
Study design and participants
The electronic medical records of patients diagnosed with HS admitted to the General Hospital of Southern Theater Command in China between 1 March 2015 and 1 September 2023 were comprehensively reviewed. The experimental procedures were approved by the Ethics Committee of the General Hospital of Southern Theater Command of PLA, Guangzhou, Guangdong Province, China (approval no. NZLLKZ2022003) and were conducted in accordance with the 1964 Helsinki Declaration and its later amendments. This retrospective study did not result in any adverse effects on human subjects. The informed verbal consent was obtained from all individual participants in the study due to objective circumstances, such as the patient’s cognitive impairment, inability to sign, and inability to meet the patients or guardians in person. The informed verbal consent was obtained by telephone follow-up to explain the process and purpose of the study and to obtain consent, which was recorded. Additionally, measures were taken to protect the patient such as concealing patient identity information and complete anonymization.
The selection of eligible patients was determined based on the following inclusion criteria: (1) age from 14 to 69 years old; (2) met the diagnostic criteria of HS described below; (3) the diagnostic criteria for HS were as follows (Leon & Bouchama, Citation2015): classic or exertional HS with a history of exposure to hot and humid weather or strenuous activity, hyperthermia (core temperature, Tc ≥ 40℃), abnormalities of the CNS, including delirium, convulsions, or coma and accompanied by multiple organ damage. The exclusion criteria were as follows: (1) age ≥ 70 or ≤ 13; (2) existing primary neurological disease before HS, such as stroke, cerebral hemorrhage, encephalitis, tumor, etc.; (3) pregnancy or breastfeeding; (4) death within 6 months after HS.
Patients were divided into the sequelae group and non-sequelae group. The sequelae were evaluated at 6 months after HS included the following: (1) symptoms that did not exist before HS; (2) abnormal symptoms and physical examination; (3) abnormal neuroelectrophysiological examination, Computed Tomography (CT)/MR imaging or laboratory findings.
The squeal group was further divided into the cerebellar injury group and the group with the other sequelae. The diagnostic criteria for the cerebellar injury were evaluated as follows at 6 months after HS: (1) cerebellar ataxia; (2) balance dysfunction; (3) cerebellar atrophy presented by craniocerebral MRI; (4) exclusion of heritability cerebellar ataxia and primary cerebellar disease.
Variables
Variables of the patients were collected upon admission, including essential demographic information, duration of hyperthermia and coma, sequential organ failure assessment (SOFA) scores, Acute Physiology and Chronic Health Evaluation-II (APACHE-II) scores, and functions of brain, liver, kidney, heart, coagulation, and immune system. The standard of diagnosis of cardiac injury is increased myocardial enzymes (twice the normal value), ST segment elevation over 3 days in electrocardiogram, circulatory failure, or reduced left ventricular systolic function in cardiac uhrasonography (Gosavi et al., Citation2016). For the diagnosis of DIC, a simple algorithm has been developed by the International Society on Thrombosis and Hemostasis and calculated with platelet count, prothrombin time, a fibrin-related marker (usually D-dimer) and fibrinogen (Levi, Citation2018).
Prognosis
The Modified Barthel Index (MBI) was used to assess activities of daily living at the 6-month follow-up after HS. The International Cooperative Ataxia Rating Scale (ICARS) and the Berg Balance Scale (BBS) were utilized to evaluate cerebellar function 6 months after HS.
Establishment of prediction model and statistics
The variables were subjected to descriptive statistical analysis. Categorical data are presented as counts and percentages, while continuous data are presented as Mean ± SEM ( ± Sx) or median (Q1 ~ Q3). The statistical analysis was conducted in six steps.
Step 1 Univariate analysis: After conducting the homogeneity of variance test, multiple comparisons among different groups were performed using one-way analysis of variance (ANOVA), followed by the Student – Newman – Keuls test. Differences between two groups were assessed through the two independent-samples Student’s t-test. Enumeration data were subjected to a Pearson χ2 test analysis.
Step 2 Logistic regression analysis (LRA): Risk factors with a p-value <0.10 obtained in Step 1 were included in the LRA using a stepwise regressive maximum likelihood ratio method. The criteria for inclusion were set at 0.05, while the exclusion criterion was set at 0.10. The inspection level (α) was set at 0.05 to further identify independent risk factors associated with sequelae of HS in patients. If there were continuous variables present in the first step LRA model, they were transformed into categorical variables. Results from the LRA are given as odds ratios (ORs) with 95% confidence interval.
Step 3 The logistic regression model: The entry method was employed to incorporate all independent risk factors identified in step 2. The inclusion criterion was set at 0.05, while the exclusion criterion was set at 0.10. A test level (α) of 0.05 is used to determine the final logistic regression model. To facilitate clinical application, the regression coefficient β value of each risk factor in the logistic regression equation was divided by the smallest coefficient (βm). The resulting new coefficients were rounded to integers and assigned as scores for each respective risk factor.
Step 4 The accuracy and consistency of the evaluation of the prediction scoring system: The accuracy of the predictive scoring model was assessed using the area under the ROC curve (AUC) and categorized into four levels: AUC ≤ 0.5 for no predictive value, 0.5≤AUC <0.7 for average predictive ability, 0.7≤AUC <0.9 for high predictive power, and AUC ≥ 0.9 for extremely high predictive power (Lemeshow & Hosmer, Citation2009). The consistency of the prediction scoring model was evaluated through Hosmer-Lemeshow goodness-of-fit test with a p-value <0.05, indicating poor consistency and > 0.05 indicating good agreement.
Step 5 Internal validation: The 1000 bootstrapping sample method was employed for internal validation of the established predictive scoring model. This involved creating a new random dataset (bootstrapping sample dataset) by resampling the original data with replacement. The statistics distribution of the bootstrapped sample dataset would be approximately equal to that of the original sample statistics. The C-index and a 95% confidence interval from the bootstrapped sample dataset was utilized to assess the stability of our predictive scoring model.
Step 6 External verification: The data from additional 64 HS patients at the General Hospital of Southern Theater Command were utilized for external validation.
All statistical analyses were performed using SPSS v25.0 (IBM Corp, Los Angeles, CA, USA) and R statistical software (R version 4.3.2). p value < 0.05 was considered statistically significant.
Findings
Baseline characteristics
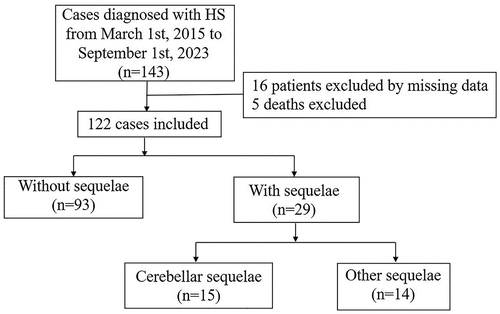
A total of 143 patients with HS were identified, and 99.3% of them presented with exertional HS. Sixteen cases were excluded due to incomplete data, and five deaths occurring within 6 months after the onset of HS were also excluded. Therefore, a total of 122 cases were included in the analysis. There are 29 (23.77% of 122 patients) patients in the sequelae group, 93 (76.23% of 122 patients) patients in the none-sequelae group, 15 (12.30% of 122 patients) patients in cerebellar sequela group and 14 (11.48% of 122 patients) patients in non-cerebellar sequela group (). The average age was 24.22 ± 0.79 years, and 119 (97.54%) of them were men. Underlying diseases identified among the cohort were hypertension (two patients, 1.64%) and ankylosing spondylitis (1 patients, 0.82%). The proportion of patients presenting with severe coma (3≤Glasgow Coma Scale, GCS<8) upon admission is 16.4% (20/122), while moderate coma (9≤GCS<12) accounts for 6.6% (8/122) and mild brain injury (13≤GCS<14) represents 13.1% (16/122).
The prevalence of sequelae following HS was 22.37%. These sequelae included neurological sequelae (18.03% of HS, constituting 75.86% of the total sequelae), hypertension (1.64% of HS, 6.9% of the sequelae), male infertility (1.64% of HS, 6.9% of the sequelae), depressive symptoms (1.64% of HS, 6.9% of the sequelae) and abnormal renal and hepatic function (0.82% of HS, 3.45% of the sequelae). The neurological sequelae comprised cerebellar dysfunction (12.3% of HS, accounting for 51.72% of the sequelae), quadriplegia (2.46% of HS, accounting for 10.34% of sequelae), epilepsy (1.64% of HS, 6.9% of the sequelae) and cognition impairment (1.64% of HS, 6.9% of the sequelae). Cerebellar dysfunction constituted the most prevalent (68.18%) neurological sequelae.
Prognosis
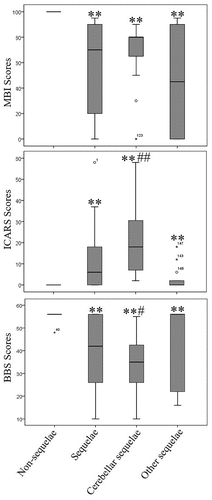
The mean MBI scores for the group with sequelae, cerebellar injury group, and other sequelae group were 57.6 ± 6.68, 68 ± 6.41, and 46.43 ± 11.55, respectively, which were significantly lower compared to the non-sequelae group (100 ± 0) (p < 0.01). The ICARS scores of the cerebellar injury group were 20.4 ± 4.13, significantly higher than both the non-sequelae group (0 ± 0) and the other sequelae group (3.17 ± 1.59) (p < 0.01). The ICARS scores of the sequelae group (12.74 ± 2.81) were also higher than the non-sequelae group (p < 0.01). The BBS scores of the cerebellar injury group were 33.8 ± 3.37, lower than both the non-sequelae group (55.9 ± 0.09) and the other sequelae group (44.9 ± 4.65) (p < 0.01). The BBS scores of the sequelae group (39.1 ± 2.98) were lower than that of the non-sequelae group (55.9 ± 0.09) (p < 0.01). ()
Figure 2. Prognosis and balance function assessments of patients with different sequelae after heatstroke.
Univariate analysis of risk factors for sequelae and cerebellar sequelae
Compared to the non-sequelae group, the sequelae group exhibited advanced age, a prolonged duration of hyperthermia and coma, lower GCS scores, elevated body temperature (Tc), as well as increased incidence of hypotension, gastrointestinal bleeding, MODS, cardiac injury, coagulation disorder, rhabdomyolysis, and metabolic acidosis upon admission. Patients with sequelae were presented with higher levers of serum creatine kinase (CK), high-sensitivity cardiac troponin T (hs-cTnT), aspartate transaminase (AST), total bilirubin, lactate dehydrogenase (LDH), standard bicarbonate (SB), creatinine and cluster of differentiation 14+ (CD14+) levels, longer prothrombin time (PT) and activated partial thromboplastin time (APTT), lower lymphocyte count, PLT count, red blood cell-specific volume (HCT) and the ratio of human leukocyte antigen-DR (HLA-DR)/CD14, increased D-dimer, SOFA scores, and APACHE-II scores compared to those without sequelae significantly (P < 0.05). Compared with the other sequelae group, the group of cerebellar sequelae exhibited significantly lower GSC scores and systolic pressure, higher levels of serum ALT, CD14+ and NSE, prolonged PT, lower levers of serum actual bicarbonate, standard bicarbonate, lymphocyte count, PLT count and HCT, increased D-dimer, and SOFA scores on admission (P < 0.05). ()
Table 1. Univariate analysis of the risk factors for the sequelae in heatstroke patients.
Logistic regression analysis and goodness of fit analysis
The results of the multivariate analysis revealed that advanced age, coma, and an APACHE-II score > 10 were identified as independent risk factors for the development of sequelae following HS (). The AUC values of age, coma, and APACHE-II score > 10 were 0.662 (95%CI: 0.571–0.745, p = 0.001) with a sensitivity of 41.38% and specificity of 89.25%, 0.883 (95%CI: 0.812–0.934, p < 0.001, sensitivity = 86.21%, specificity = 90.32%), 0.834 (95%CI: 0.756–0.895, p < 0.001, sensitivity = 68.97%, specificity = 97.85%), respectively (). The AUC for the combined three risk factors was 0.941 (95% CI: 0.884 ~ 0.976, p < 0.001). Predicting sensitivity (Se) = 0.8966 (negative predicting accuracy rate) and specificity (Sp) = 0. 8925 (positive predicting accuracy rate) (). The p value obtained from the Hosmer-Lemeshow goodness-of-fit test was 0.976. The total accuracy rate was 0.8000. The predictive efficacy of comprehensive indicators for sequelae in HS patients surpasses that of individual risk factors.
Figure 3. ROC curve of risk factors to sequelae and cerebellar dysfunction of heatstroke patients and internal/external validation of the risk prediction score system.
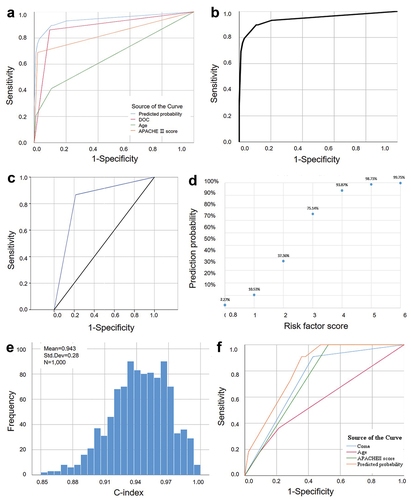
Table 2. Multivariate analysis of relevant factors of the sequelae in patients with heatstroke.
Compared with the other sequelae group, the risk factor associated with cerebellar sequelae was a SOFA score > 5 (p = 0.003). The χ2 value of Hosmer-Lemeshow test for the risk factor of cerebellar sequelae was 3.225 (p = 0.863). The AUC value of the risk factor was 82.6% (95% CI 0.664–0.989, p = 0.003). The sensitivity of the optimal cutoff value was 86.7%, and the specificity was 78.6% ().
Modeling of a prediction system
The risk factors were reassigned using logistic regression coefficients to establish a scoring system for prognosticating the risk of sequelae. The scoring system ranges from 0 to 6 points, with 1 point awarded for individuals aged 31–45 and 2 points for those ≥ 46. Additionally, a score of 2 is given for coma status and an APACHE-II score ≥ 10. The total possible score is therefore 6 points (). The sequelae of probability and risk are directly proportional, with a higher risk score indicating an increased likelihood of sequela occurrence ().
Table 3. The prediction scale of risk factors for sequelae of heat stroke.
To enhance the clinical applicability of the prediction model, we categorized the score into three grades based on clinical experience and previous literature reports: Scores 0–1 were classified as low-risk, indicating an average incidence of sequelae at 4.6%; Scores 2–3 were classified as medium-risk, corresponding to an average incidence rate of 40.0%; Scores 4–6 were classified as high-risk, with an average incidence rate reaching 95.0% (). For example, a patient aged 36 years old (part 1 of the scale = 1), presenting with coma (part 2 of the scale = 2) and an APACHE II score > 10 (part 3 of the scale = 2) upon admission, was diagnosed with HS, and the total score was 5 (1 + 2 + 2). The total score was classified under category III, indicating a significant likelihood (95%) for sequelae development following HS.
Table 4. Incidence of the sequelae after heatstroke corresponding to different risk grades.
Internal and external validation of the model
After conducting bootstrapping sampling and internal verification, the results indicated a high level of discrimination with a score of C-index = 0.942 (95%CI: 0.847 ~ 0.981) (). The external validation included additional 64 patients diagnosed with HS and admitted to the General Hospital of Southern Theater Command in China between 1 March 2012 to 28 February 2015. There are 42 (65.63% of 64 patients) patients without sequelae and 22 (34.38% of 64 patients) patients with sequelae, among whom 18 patients had cerebellar sequelae while the remaining four patients had other types of sequelae (One case each exhibited cognitive impairment, common peroneal nerve injury, renal dysfunction, and hypertension). The average age was 27.05 ± 1.54 years, and 60 (93.75%) of them were men. The AUC values of age, coma, and APACHE II score were 0.577 (95%CI: 0.447 ~ 0.699, p = 0.218, the sensitivity was 36.36%, the specificity was 78.57%), 0.740 (95%CI: 0.615 ~ 0.842, p < 0.001, the sensitivity was 90.91%, the specificity was 57.14%), 0.738 (95%CI: 0.613 ~ 0.840, p < 0.001, sensitivity was 100.00%, specificity was 47.62%), respectively. Combining the three risk factors yielded an AUC value of 0.816 (95%CI: 0.699 ~ 0.902, p < 0.001), with a sensitivity of 90.91% and specificity of 64.29% ().
Discussion
In this study, a predictive model was developed to assess the incidence of sequelae in patients with HS at an early stage. The presence of advanced age, coma, and a higher APACHE-II score upon admission were associated with an increased incidence of sequelae in patients. Patients who experienced sequelae after HS had a poorer prognosis compared to those without sequelae. Furthermore, in comparison to patients with other sequelae, HS patients with cerebellar dysfunction exhibited higher SOFA scores upon admission, elevated ICARS levels, and decreased BBS at 6 months post-HS.
The long-term outcome indicated that HS had a profound impact on neurological and cardiovascular functions (Nzvere et al., Citation2020). About 22–33% of the survivors were discharged with sustained or worsened neurologic disability (Mahri & Bouchama, Citation2018). The results of this study are consistent with the fact that 18.03% of the survivors experienced neurological sequelae, with cerebellar dysfunction being the most prevalent. The occurrence of coma was identified as a significant risk factor for the development of sequelae following HS in this study. HS patients with lower GCS scores and higher Tc upon admission were more likely to experience neurological sequelae (Hifumi et al., Citation2018). Patients with sequelae had longer duration of coma and hyperthermia than those without sequelae. Longer duration of hyperthermia and lower GCS score were also risk factors for cerebellar injury. Deep coma characterized by a low GCS score and prolonged duration serves as significant indicators of severe cerebral ischemia and hypoperfusion (Dunham et al., Citation2004). Hypotension, intracranial hypertension, cerebral hypoperfusion, and ischemia were observed in HS animal models (Chang et al., Citation2004). Neurons are highly vulnerable to ischemia and high temperature, leading to irreversible cell damage with permanent disability (Au et al., Citation2015; Li et al., Citation2013). The duration of hyperthermia was correlated with the extent of cell death in purkinje cells (Kosgallana et al., Citation2013). Heat may give rise to delayed vestibulopathy by damaging the vestibulocerebellum (Jung et al., Citation2017). The aforementioned factors may elucidate the correlation between coma and sequelae, particularly the cerebellar sequelae. The risk of mortality was significantly elevated in patients with a GCS score of less than 8 and a duration in a coma exceeding 24 hours. Decreased GCS score on admission was also a risk factor for 90-day mortality in patients with HS (Zhong, Wu, Liu, et al., Citation2021). The results above emphasize the necessity of closely monitoring the degree and duration of coma during the early stages of HS, as well as initiating timely brain protection measures.
The sequelae of HS were influenced by an increase in APACHE-II scores on admission, while the elevated SOFA score emerged as the sole statistically significant risk factor for cerebellar dysfunction. The APACHE II and SOFA scores are the most widely used and authoritative critical illness evaluation system in intensive care unit, which include assessments of multi-organ functions (Kądziołka et al., Citation2019). In this study, patients with sequelae following HS had more multiple-organ dysfunction upon admission, including cardiac injury, coagulation disorder, rhabdomyolysis, metabolic acidosis, and immunologic dysfunction. Firstly, coagulation dysfunction is an independent cause of death in HS (Hifumi et al., Citation2017). HS-induced coagulation dysfunction is triggered by coagulation factors, inflammatory activation, and endothelial injury (Bouchama et al., Citation2012; Hifumi et al., Citation2017). HS patients with PLT abnormalities upon admission have a lower 90-day survival rate and more MODS, including coagulation, hepatic and renal dysfunction, suggesting that coagulation disorder predicted a poor prognosis (Zhong, Wu, Ji, et al., Citation2021). Secondly, the incidence of cardiac dysfunction in patients with HS is 43.4% ~ 65.2% (Leon & Bouchama, Citation2015). HS-induced cardiac injury is associated with cardiomyocytes and endothelial cells injury caused by heat (Qian et al., Citation2004; Yang et al., Citation2014). Impairment of cardiac function could aggravate hypotension, leading to aggravating intracranial hypoperfusion and cerebral ischemia. Thirdly, the decreased ratio of HLA-DR/CD14, higher levels of CD14 and lymphocytopenia indicated a state of immune deficiency and susceptibility to infection, leading to poor prognosis. These results indicated a significant correlation between higher SOFA and APACHE II scores upon admission and long-term outcomes. Although increased SOFA scores were associated with cerebellar sequelae following HS, the mechanism underlying delayed cerebellar injury induced by HS remains unclear. The severe state of multi-organ damage indicated by the high SOFA score may potentially be associated with secondary ischemia or neuroinflammation in the cerebellum.
Notably, older patients were more likely to experience sequelae after HS. A study suggested that age 40–59 was the most prevalent age group suffering from HS (Kaewput et al., Citation2021). The age-specific distribution of HS is bimodal and peaks at 10–20 and 50–60 years of age, and age differences affected the incidence of heat-related illness (Onda & Yokota, Citation2012; Ueno et al., Citation2021). In this study, the incidence of sequelae was also influenced by age. Age is an important variable in the etiology of thermoregulatory failure, which can lead to HS and death (Durkot et al., Citation1986). The recuperation of organ function was comparatively prolonged in elderly patients following HS, potentially explaining the higher occurrence of complications in older individuals. Therefore, the prediction system incorporated advanced age, coma, and APACHE-II score and demonstrated a high level of sensitivity in both internal and external validation. The implementation of this prediction system will assist clinicians in early identification of HS patients at risk for sequelae, thereby facilitating comprehensive multi-organ treatment and functional rehabilitation.
The limitations of this study are as follows: (1) It is a retrospective single-center study. A multi-center and long-term clinical observational study will be conducted at a later stage to identify additional sequelae and risk factors following HS. (2) The limited occurrence of other sequelae precluded the possibility of conducting regression analysis in this study. Future studies will include more cases to investigate the characteristics of these remaining sequelae, such as cardiac injury or prolonged hepatic and renal dysfunction.
Conclusions
Heatstroke patients presenting with advanced age, coma, and higher APACHE-II score upon admission exhibit a greater incidence of sequelae and a poorer prognosis. Among them, those with elevated SOFA scores are more prone to experiencing cerebellar dysfunction characterized by balance disorders and ataxia.
Author contributions statement
XXN: designing, data acquisition, drafting of the original draft and revising; GCX: designing, statistical analysis, modeling and validation; YQG: data acquisition and analysis; XJX: methodology, conceptualization and analysis; JW: designing and investigation; ZFL: funding acquisition, conception and designing, revising and the final approval of the version to be published. All authors agree to be accountable for all aspects of the work.
Ethical approval statement
This study was approved by the Ethics Committee of the General Hospital of Southern Theater Command of PLA, Guangzhou, Guangdong Province, China (approval no. NZLLKZ2022003) and were conducted in accordance with the 1964 Helsinki Declaration and its later amendments. This retrospective study did not result in any adverse effects on human subjects. The informed verbal consent was obtained from all individual participants in the study due to objective circumstances, such as the patient’s cognitive impairment, inability to sign, and inability to meet the patients or guardians in person. The informed verbal consent was obtained by telephone follow-up to explain the process and purpose of the study and to obtain consent, which was recorded. Additionally, measures were taken to protect the patient such as concealing patient identity information and complete anonymization.
Acknowledgements
I would like to express my sincere gratitude to Prof. Lei Su and Rong-hao Yu for their invaluable guidance throughout the course of this research.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data available on request from authors.
Additional information
Funding
References
- Albukrek, D., Bakon, M., Moran, D. S., Faibel, M., & Epstein, Y. (1997). Heat-stroke-induced cerebellar atrophy: Clinical course, CT and MRI findings. Neuroradiology, 39(3), 195–197.
- Au, A. K., Chen, Y., Du, L., Smith, C. M., Manole, M. D., Baltagi, S. A., Chu, C. T., Aneja, R. K., Bayır, H., Kochanek, P. M., & Clark, R. S. B. (2015). Ischemia-induced autophagy contributes to neurodegeneration in cerebellar purkinje cells in the developing rat brain and in primary cortical neurons in vitro. Biochimica et Biophysica Acta, 1852(9), 1902–1911.
- Bazille, C., Megarbane, B., Bensimhon, D., Lavergne-Slove, A., Baglin, A. C., Loirat, F. W. P., Mikol, J., & Gray, F. (2005). Brain damage after heat stroke. Journal of Neuropathology & Experimental Neurology, 64(11), 970–975.
- Bouchama, A., Al-Mohanna, F., Assad, L., Baturcam, E., Eldali, A., Owaidah, T., & Dehbi, M. (2012). Tissue factor/factor VIIa pathway mediates coagulation activation in induced-heat stroke in the baboon. Critical Care Medicine, 40(4), 1229–1236.
- Chang, C. K., Chiu, W. T., Chang, C. P., & Lin, M. T. (2004). Effect of hypervolaemic haemodilution on cerebral glutamate, glycerol, lactate and free radicals in heatstroke rats. Clinical Science, 106(5), 501–509.
- Dunham, C. M., Ransom, K. J., Flowers, L. L., Siegal, J. D., & Kohli, C. M. (2004). Cerebral hypoxia in severely brain-injured patients is associated with admission Glasgow Coma Scale score, computed tomographic severity, cerebral perfusion pressure, and survival. Journal of Trauma, 56(3), 482–489.
- Durkot, M. J., Francesconi, R. P., & Hubbard, R. W. (1986). Effect of age, weight, and metabolic rate on endurance, hyperthermia, and heatstroke mortality in a small animal model. Aviation, Space, and Environmental Medicine, 57(10 Pt 1), 974–979.
- Gosavi, S., Tyroch, A. H., & Mukherjee, D. (2016). Cardiac trauma. Angiology, 67(10), 896–901.
- Hifumi, T., Kondo, Y., Shimazaki, J., Oda, Y., Shiraishi, S., Wakasugi, M., Kanda, J., Moriya, T., Yagi, M., Ono, M., Kawahara, T., Tonouchi, M., Yokota, H., Miyake, Y., & Shimizu, K. (2017). Prognostic significance of disseminated intravascular coagulation in patients with heat stroke in a nationwide registry. Journal of Critical Care, 44(306–311), 306.
- Hifumi, T., Kondo, Y., Shimizu, K., & Miyake, Y. (2018). Heat stroke. Journal of Intensive Care, 6, 30. https://doi.org/10.1186/s40560-018-0298-4
- Jung, I., Choi, S.-Y., Kim, H.-J., & Kim, J.-S. (2017). Delayed vestibulopathy after heat exposure. Journal of Neurology, 264(1), 49–53.
- Kądziołka, I., Świstek, R., Borowska, K., Tyszecki, P., & Serednicki, W. (2019). Validation of APACHE II and SAPS II scales at the intensive care unit along with assessment of SOFA scale at the admission as an isolated risk of death predictor. Anaesthesiology Intensive Therapy, 51(2), 107–111. https://doi.org/10.5114/ait.2019.86275
- Kaewput, W., Thongprayoon, C., Petnak, T., Cato, L. D., Chewcharat, A., Boonpheng, B., Bathini, T., Vallabhajosyula, S., & Cheungpasitporn, W. (2021). Inpatient burden and mortality of heatstroke in the United States. International Journal of Clinical Practice, 75(4), e13837. https://doi.org/10.1111/ijcp.13837
- Kosgallana, A. D., Mallik, S., Patel, V., & Beran, R. G. (2013). Heat stroke induced cerebellar dysfunction: A “forgotten syndrome. World Journal of Clinical Cases, 1(8), 260–261.
- Lawton, E. M., Pearce, H., & Gabb, G. M. (2019). Environmental heatstroke and long-term clinical neurological outcomes: A literature review of case reports and case series 2000–2016. Emergency Medicine Australasia, 31(2), 163–173. https://doi.org/10.1111/1742-6723.12990
- Lemeshow, S., & Hosmer, J. (2009). Logistic regression analysis: Applications to ophthalmic research. American Journal of Ophthalmology, 147(5), 766–767.
- Leon, L. R., & Bouchama, A. (2015). Heat stroke. Comprehensive Physiology, 5(2), 611–647.
- Levi, M. (2018). Pathogenesis and diagnosis of disseminated intravascular coagulation. International Journal of Laboratory Hematology, 40(Suppl 1), 15–20.
- Li, C.-W., Lin, Y.-F., Liu, T.-T., & Wang, J.-Y. (2013). Heme oxygenase-1 aggravates heat stress-induced neuronal injury and decreases autophagy in cerebellar purkinje cells of rats. Experimental Biology and Medicine, 238(7), 744–754.
- Mahri, S. A., & Bouchama, A. (2018). Heatstroke. Handbook of Clinical Neurology, 157, 531–545. https://doi.org/10.1016/B978-0-444-64074-1.00032-X
- Moshe, R.-A., Mony, S., Shalom, H., Moshe, G., & Iftah, B. (2007). Unique persistent neurological sequelae of heat stroke. Military Medicine, 172(6), 603–606.
- Nzvere, F. P., Tariq, E., Nishanth, K., Arshid, A., & Cancarevic, I. (2020). Long-term cardiovascular diseases of heatstroke: A delayed pathophysiology outcome. Cureus, 12(8), e9595. https://doi.org/10.7759/cureus.9595
- Onda, H., & Yokota, H. (2012). Risk factors of heatstroke. Nihon Rinsho, 70(6), 947–951.
- Qian, L., Song, X., Ren, H., Gong, J., & Cheng, S. (2004). Mitochondrial mechanism of heat stress-induced injury in rat cardiomyocyte. Cell Stress & Chaperones, 9(3), 281–293.
- Ueno, S., Hayano, D., Noguchi, E., & Aruga, T. (2021). Investigating age and regional effects on the relation between the incidence of heat-related ambulance transport and daily maximum temperature or WBGT. Environmental Health and Preventive Medicine, 26(1), 116. https://doi.org/10.1186/s12199-021-01034-z
- Yang, Y., Duan, W., Jin, Z., Yi, W., Yan, J., Zhang, S., Wang, N., Liang, Z., Li, Y., Chen, W., Yi, D., & Yu, S. (2014). JAK2/STAT3 activation by melatonin attenuates the mitochondrial oxidative damage induced by myocardial ischemia/reperfusion injury. Journal of Pineal Research, 55(3), 275–286.
- Yaqub, B., & Al Deeb, S. (1998). Heat strokes: Aetiopathogenesis, neurological characteristics, treatment and outcome. Journal of the Neurological Sciences, 156(2), 144–151.
- Zhong, L., Wu, M., Ji, J., Wang, C., & Liu, Z. (2021). Association between platelet levels on admission and 90-day mortality in patients with exertional heatstroke, a 10 years cohort study. Frontiers in Medicine, 8, 716058. https://doi.org/10.3389/fmed.2021.716058
- Zhong, L., Wu, M., Liu, Z., Liu, Y., Ren, G., Su, L., & Liu, Z. (2021). Risk factors for the 90-day prognosis of severe heat stroke: A case-control study. Shock, 55(1), 61–66. https://doi.org/10.1097/SHK.0000000000001589